International Journal of Anatomical Sciences 2012, 3(2): 26-32
Research Paper
Bacopa monniera a Potent Neuroprotector Against Transient Global
Cerebral Ischemia Induced Hippocampal Damage and Memory Function
Ramesh Kumar R, Kathiravan K, Muthusamy R.
Department of Anatomy, Dr.A.L.M. Post Graduate Institute of Basic Medical Sciences, University of Madras, Taramani campus Chennai 600 113, Tamil Nadu, India.
Key words: Global cerebral ischemia, Bacopa monniera, Radial arm maze
Abstract: Cerebral ischemia is the major complication associated with the vascular health, next to cardiovascular disease, resulting in irreversible damage to the brain tissues and permanent loss of specific brain functions. Hippocamous is one of the most vulnerable regions of brain for global cerebral ischemic insult with impaired memory. Bacopa monniera (BM) is a well known traditional herbal medicine extensively used as an adjuvant to enhance cognitive development, as well to treat various neurological disorders. The present study emphasis the effect of the prophylactic administration of BM against hippocampal damage and spatial learning induced by Transient Global Cerebral Ischemia (TGCI) by 4VO method in wistar albino rats. Earlier the rats were trained for spatial navigation task in an eight arm radial maze for 10 days and were simultaneously treated with the ethanolic extract of BM, at a dose of 100mg/kg body weight/ ml /day orally for 30 days and then the animals were subjected to TGCI for 15 minutes. After TGCI the rats were tested for the retention of spatial learning and memory in the radial arm maze and the reference memory error, working memory error and latency were determined. The normal pyramidal cell density in the CA1 region of dorsal hippocampus was also counted. The outcome of this study suggests that BM could protect pyramidal cell damage of CA1 region of hippocampus and retention of hippocampal associated spatial learning and memory in the rats pretreated with BM extract, this finding confirms the neuroprotective role of BM against cerebral ischemia.
Transient global cerebral ischemia (TGCI) is a major consequence of cardiac arrest resulting in sudden circulatory block to complete brain. TGCI leads to selective damage of neurons in the vulnerable brain regions, especially CA1 region of hippocampus, few cortical neurons located in layer 3, 5 and 6 and few striatal neurons (Hartman et al, 2005; Kubo et al, 2009) and resulting in long term learning and memory deficits( Farkas et al, 2001). Among the brain regions hippocampus and its subfields are more vulnerable to TGCI and an extensive neuronal death was found in the CA1 region of rodent hippocampus starting from 2 to 4 days after ischemia and reaching a maximal effect within 1–2 weeks dependending on the duration of the ischemic insult (Bendel et al., 2005; Colbourne et al., 1999; Pulsinelli et al.,1982; Smith et al., 1984). The pronounced deletion of the CA1 pyramidal neurons is associated with severe impairments of hippocampal-dependent brain functions, such as spatial learning and memory (Bendel et al., 2005; Block, 1999; Hartman et al.,2005).
Several experimental studies have been carried out to combat the deleterious effect of cerebral ischemia and to promote a better therapeutic module which can benefit the brain function by protecting the neurons against the ischemic damage. Natural products, especially medicinal plants, could be an ideal source to develop safe and effective agents for neuroprotection against cerebral ischemia (Kim, 2005). Bacopa monniera (BM) has been a major constituent of traditional Indian medicine and its encrypts were seen in Charaka Samitha, a medicinal book of 5th century A.D. The biological effects of BM are also documented in various other traditional as well as scientific literatures. It is a rich source of antioxidant and has been reported to reduce oxidation of fats in the blood stream (Stough et al., 2001). It has been used for centuries in traditional medicinal practice to help benefit against epilepsy, memory capability, to increase concentration and to reduce stress-induced anxiety, It is also listed as a nootropic, a drug that enhances cognitive ability (Singh and Dhawan, 1992; Dhawan and Singh 1996; Russo, et al, 2006).
This present study is aimed to understand the effect of BM on the TGCI induced learning impairment and memory disorder and the neurodegenerative consequences.
Materials and Methods
Animals
Twelve healthy adult Male Wistar albino rats weighing about 200 – 250 gms were used for this study. They were maintained in an optimum environment of constant temperature (21◦C), humidity and 12 hours of day and night cycle. Animals were fed with standard food pellets and water ad libitum. The experiments were conducted in accordance with the standard guidelines of the Institutional Animal Ethical Committee (IAEC).
Transient Global Cerebral Ischemic Model
Forebrain ischemia was performed using the four-vessel occlusion method (4VO) i.e. Bilateral vertebral and common carotid artery, as previously described by Pulsinelli and Brierley (1979); Lee et al (2012). Briefly the animals were anesthetized with Ketamine & Xylazine (80 mg and 10 mg/kg body weight, intraperitonealy). The surgical procedure consists of two steps, Step 1. Includes electro-cauterization of the bilateral vertebral arteries on day one, followed by Step 2. Bilateral common carotid artery occlusion for 15 minutes using micro vascular clips (FST, USA) on next day. For sham group the above mentioned surgical procedures were performed, except bilateral common carotid artery occlusion. This study includes three experimental groups i.e. (i) Sham, (ii) TGCI and (iii) Pretreatment of Bacopa monniera (BM) for 30 days followed by TGCI. After surgical procedures rats were maintained for a week under proper post operative care.
Bacopa monniera (BM) extract
Ethanolic extract of BM with 40% Bacoside A (The active compound responsible for its cognitive function) was procured from Bayer’s, Bangalore, India. BM extract was dissolved in distilled water and made as a suspension. BM suspension was administered to the animals for 30 days orally at a dose of 100 mg/Kg body weight before they were exposed to TGCI.
Radial arm maze Test
To assess the spatial learning and memory in rats the radial arm maze test was performed. The radial arm maze (RAM) consisted of an octagonal platform (26cm) with eight arms (42 x 12 cm) and placed 60cm above the floor. Each animal was trained individually prior to the surgery, the training module started with three baited arms (numbers 3, 5 and 7) and the remaining arms were un-baited. The trial continued until all four baits had been consumed or until 5 minutes had elapsed. Reference Memory Errors (RME) i.e. Entering an arm that was not baited and Working Memory Errors (WME) i.e. Re-entering an arm containing food and latency were assessed after one week of regular training and post surgical assessment was made on day 1 & 7.
Histology
At the end of post operative experimental trials the rats were euthanized with Thiopentone sodium 80mg/Kg body weight and transcardially perfused with 4% paraformaldehyde in phosphate buffered saline. The animals were decapitated and brains were removed. The fore brain was processed and embedded in paraffin wax and the tissue blocks were sectioned in to 10 micron thick sections using a rotary microtome (Weswox, India). The sections obtained were stained using Cresyl fast violet (CFV). Density of normal neuronal population in the granular cells of the CA1 region within the 400µm2 of bilateral dorsal hippocampus was counted at defined coronal in blinded fashion with reticule incorporated eyepiece at a magnification of 200X using a light microscope. A quantitative estimation of cell damage was made by direct visual counting of apparently normal neurons in the bilateral CA1 area of all the three animal groups within the reticule area. Cells showing dark cytoplasm and shrinkage were not counted.
Statistical analysis
All data were expressed as mean ± standard error mean (SEM) and the statistical analysis of the results was performed by one-way analysis of variance (ANOVA) followed by turkey’s test using SPSS. P values ≤ 0.05 was considered significant.
Observations
The effect of pretreatment of BM on the transient Global cerebral ischemic rats was well documented in this study through spatial learning and memory behavior in RAM task and the histological assessments.
Radial arm maze (RAM) Test
In this study the hippocampal associated spatial learning and memory of the rats was assessed in the radial arm maze. On day 1 after 4VO in the RAM task the group II rats showed a significant increase in the reference memory error (RME) i.e. 10.5 ± 0.86 (Mean ± SEM) than the group I (1.25 ± 0.25), similarly the group III rats also showed a significant increase (7.75 ± 0.85), when compared with group I. The group III rats showed moderate decrease in RME when compared with group II, but statistically non significant. On Day 7 after4VO and reperfusion the RME of group II (5.75 ± .025) was significant higher than group I (0.75 ± .25) and RME of group III (4.0± 0.40) was significantly less, when compared with group II (Fig 1a).
Fig. 1a Reference Memory Error Assessment
Using Radial Arm Maze
The working memory error (WME) on day1 after 4VO and reperfusion was significantly higher in group II (2.75±0.25) and group III (1.75 ± 0.25) when compared to the group I rats, which performed without any errors. The group III rats showed a significant decrease in WME when compared with group II. On day 7 after lesion the WME of group II (1.75±0.50) and group III (1.50±0.59) was significantly higher when compared to group I, which performed without any WME. The group III showed a marginal reduction in WME when compared to group II, But statistically non significant (Fig 1b).
Fig. 1b Working Memory Error Assessment
Using Radial Arm Maze
Latency (The time taken by the rats to complete the RAM task) on day1 was significantly higher in group II (4.93±0.20) and group III (2.81±0.27) when compared to group I (0.84±0.21). The group III rats showed a significant reduction in latency, when compared with group II. On assessment day 7 group II (2.51±0.37) alone showed a significant increase in latency, when compared to group I (0.92±0.15). The group III (1.32±0.75) rats showed a significant decrease when compared to group II (Fig. 1c).
Histology
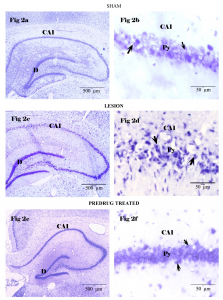
The brain sections stained with CFV stain obtained from Group I rats 7 days after bilateral vertebral artery occlusion, we could observe intact morphology of bilateral hippocampus (Fig 2a). The pyramidal neurons of CA1 region demonstrated a normal profile with pale nucleus and prominent nissl granules in the cytoplasm, without any shrinkage (Fig 2b). In the Group II rat brain sections the hippocampus demonstrated major alteration in the neuronal profile of CA1 region (Fig 2c), the pyramidal cells of CA1 region showed an increase in the number of dead, darkly stained shrunken pyramidal cells with pyknotic nucleus. The number of normal pyramidal cell population was drastically less (Fig 2d). The brain sections of predrug treated rats (Group III), 7 days of cerebral reperfusion after 4VO in CFV stain showed a decreased number of shrunken cells, damaged neurons in the CA1 region of hippocampus and the proportion of normal cell in the pyramidal region was comparatively higher than the lesion group (Fig 2e, 2f)
Fig. 1c Latency exhibited by animals of various groups as assessed using Radial Arm Maze
*p<0.05 compared to sham, #p<0.05 compared to lesion group.
The count of normal pyramidal neurons within the 400µm2 of defined CA1 region of bilateral hippocampus in the group I rats was 118.31± 1.46 (Mean ± SE). The cell count in group II (43.43 ± 1.33) and in the group III (72.37± 1.29) was significantly reduced when compared with group I rats. The group III rats showed significant increase in the number of CA1 pyramidal neurons, when compared with group II (Fig.3).
Discussion
The transient global cerebral ischemia by 4VO methods in Wistar albino rat have induced a progressive bilateral damage to the CA1 pyramidal neurons of the rat hippocampus and have significantly reduced the percentage of surviving pyramidal neurons (Pulsinelli et al., 1982). The outcome of this present study correlates with the above data resulting in permanent selective damage to the CA1 region of the hippocampus with retarded performance in the radial arm maze task.
Fig. 2 Histological appearance of hippocampus in animals of experimental groups
Cresyl fast violets stained brain sections, Fig 2a, 2b represents Group I (Sham), the arrow indicates the prominent surviving neurons. Fig 2c, d represents Group II (Lesion) arrow indicates the dead shrunken neurons with pyknotic nucleus. Fig 2e, f represents group III (BM treated) arrow represents few dead neurons. Py – pyramidal cell layer, D- Dentate gyrus.
Fig. 3 Number of surviving pyramidal neurons in stress, exitotoxicity, glial cells mediated inflammatory reactions, apoptotic cell death, etc (Mattson et al., 2000; Lo et al., 2003). The ethanolic extract of BM was found to inhibit calcium influx via voltage and receptor operated calcium channels of cell membrane in the intestinal smooth muscles.
The extent of the pyramidal cell damage by counting the surviving neurons in predetermined CA1 region hippocampus. *p<0.05 compared to sham, #p<0.05 compared to lesion group.
BM a traditional medicinal plant, which was well known as a potent nervine tonic, enhancing cognitive function and well documented in the studies conducted in normal subjects both experimental and clinical trials (Singh et al., 1997). Apart from cognitive enhancing property BM’s potential on various disease conditions, especially neurological disorders were experimentally proved (Russo et al., 2003; Jyoti et al 2006) considering this versatile role of BM on brain functions especially on cognitive function, an effort was made through this study to understand whether the prophylactic administration of BM for 30 days prior to the induction of TGCI in wistar albino rat. The BM treated rats could retain the spatial memory and could execute the RAM task with less error and reduced latency than the untreated rats. The histological observations also showed that the density of surviving neurons in the CA1 region of BM treated rats was significantly higher than the lesion group. By this study it was ascertained that pretreatment of BM made tremendous contribution to protect the pyramidal cells from the adverse effect of TGCI and supported to retain the spatial memory in the RAM task.
Guinea pig (Dar et al., 1999). During ischemic and reperfusion phase, a significantly increased levels of several free- radical species are generated in brain, that degrade cell and capillary membranes have been postulated (Safar, et al, 1986). In the previous study, B. monniera has been documented to provide neuroprotection against cigarette smoke induced apoptosis (Anbarasi et al, 2006) and aluminium induced oxidative stress (Jyoti et al, 2007). Neuroprotective role of BM was also demonstrated in transient bilateral internal carotid artery occluded rats by administering variable dosages (Saraf, et al, 2010).
This neuroprotective role of BM in TGCI induced 4 vessel model of Wistar albino rat may be due to its rich antioxidant resource it posses (Stough et al., 2001; Bhattacharya, et al., 2000) might have controlled the free radical generated during cerebral ischemia and prevented neuronal death or its active compound might have evoked an effective resistance shield against various other mechanism which operates the ischemic cascade.
Conclusion
In conclusion, this study has given a viable data that BM, traditionally well known drug for memory enhancement could also protect one of the most vulnerable population of brain regions, the hippocampus against the deleterious effect of transient global cerebral ischemia and retained the hippocampus mediated memory functions. The core mechanism of BM which works against the ischemic cascade operated by TGCI was not completely understood through this study. So a future detailed investigation could explore the unknown potentiality of Bacopa monniera.
References
Anbarasi K, Vani G, Balakrishna K, Devi CS (2006).
Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci,78:1378–1384.
Bendel O, Alkass K, Bueters T, von Euler M, von Euler G (2005) Reproducible loss of CA1 neurons following carotid artery occlusion combined with halothane-induced hypotension. Brain Res, 1033: 135–142.
Bhattacharya SK, Bhattacharya A, Kumar A, Ghosal S (2000) Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytotherapy Res, 14:174–179.
Colbourne F, Li H, Buchan AM (1999) Continuing post-ischemic neuronal death in CA1. Influence of ischemia duration and cytoprotective doses of NBQX and SNX-111 in rats. Stroke, 30: 662–668.
Dar A, Channa S (1999) Calcium antagonistic activity of Bacopa monniera on vascular and intestinal smooth muscles of rabbit and guinea pig. J Ethnopharmacol, 73: 893-900.
Dhawan BN, Singh HK (1996) Pharmacology of ayurvedic nootropic Bacopa monniera, Abstract No. NR 59. International convention of Biology and Psychiatry.
Farkas E, Luiten PG (2001) Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol, 64: 575–611.
Hartman RE, Lee JM, Zipfel GJ, Wozniak DF (2005) Characterizing learning deficits and hippocampal neuron loss following transient global cerebral ischemia in rats. Brain Res, 1043: 48–56
Jyoti A, Sethi P, Sharma D (2007) Bacopa monniera prevents from aluminium neurotoxicity in the cerebral cortex of rat brain. J Ethnopharmacol, 111:56–62.
Jyoti A, Sharma D (2006) Neuroprotective role of Bacopa monniera extract against aluminium induced oxidative stress in the hippocampus of rat brain. Neurotoxicology, 27: 451–457.
Kim H (2005) Neuroprotective herbs for stroke therapy in traditional eastern Medicine. Neurol Res, 27: 287-301.
Kubo K, Nakao S, Jomura S, Sakamoto S, Miyamoto E, Xu Y, Tomimoto H, Inada T, Shingu K (2009)Edaravone, a free radical scavenger, mitigates both gray and white matter damages after global cerebral ischemia in rats. Brain Res, 1279: 139 –146.
Lee D, Park J, Yoon J, Kim MY, Choi HY, Kim H (2012) Neuroprotective effects of Eleutherococcus senticosus bark on transient global cerebral ischemia in rats. J Ethnopharmacol, 139: 6–11.
Lo EH, Dalkara T, Moskowitz M (2003) Mechanisms, challenges and opportunities in stroke, Nature Rev – Neurosci, 4: 399–415.
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nature Rev- Mol Cell Bio, 1:120–130.
Pulsinelli WA, Brierley JB, Plum F (1982) Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol, 11:491-498.
Pulsinelli WA, Brierley JB (1979) A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke, 10:267–72.
Russo A, Borrelli F (2005) Bacopa monniera, a reputed nootropic plant: an overview. Phytomedicine, 12: 305-317.
Russo A, Borrelli F, Campisi A, Acquaviva R, Raciti G, Vanella A (2003) Nitric oxide-related toxicity in cultured astrocytes: effect of Bacopa monniera. Life Sciences, 73:1517–1526.
Safar P (1986) Cerebral resuscitation after cardiac arrest: a review. Circulation, 74: 38-53.
Singh HK, Dhawan BN (1997) Neuropschyco- pharmacological effect of the Ayurvedic nootropic Bacopa monniera. Linn (Brahmi). Indian J Pharmacol, 29: 359–365.
Stough C, Lloyd J, Clarke J (2001) The chronic affects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacol, 156: 481-484