International Journal of Anatomical Sciences 2013, 4(1):12-21
Research Paper
Functional Topography of Human Face
Balaji Karuppaiah, Perumal Saraswathi.
Department of Anatomy, Saveetha Medical College & Hospital, Thandalam, Chennai – 602 105, Tamil Nadu, India.
Key words: Functional magnetic resonance imaging (fMRI), Blood Oxygen Level Dependent (BOLD) Technique, Echo planar imaging (EPI)
Abstract: Over yonks, motor cortex were studied by stimulatory method, with the advent of functional Magnetic Resonance Imaging , a new road map is created in studying motor cortex of human brain by non- invasive technique. In this study functional topography of human face is constructed using Blood Oxygen Level Dependent Technique .Instructions were given to the normal individuals to perform various paradigms involving different parts of the body and the corresponding cortex of the brain are represented as glowing areas. The amount of motor cortex involved is directly proportional to the density of innervations and not the area of the body surface.
Functional magnetic resonance imaging (fMRI) visualizes the active process in brain functions. It shows good correlation of neuronal function by shift in blood oxygenation following neuronal activity using Blood Oxygen Level Dependent (BOLD) Technique. Using non-invasive fMRI method, it is possible to localize functional brain activation in normal individuals with an accuracy of spatial and temporal resolution. Though numerous technical challenges remained, fMRI was increasingly becoming a key method for understanding the topographical organization of human brain (Cohen et al. 1994). The aim of the present study was to find the somatotopic representation of face in the cerebral hemisphere.
Materials and Methods
The present study examined the activation maps of face in normal adults. Institutional Review Board (IRB) and Ethical Committee (EC) approved the study. Each subject (patient) gave written informed consent. fMRI data were collected from 6 healthy volunteers aged above 25 yrs. Using Siemens 1.5 T (70mm wide bore MRI), fMRIs were acquired in active and rest states by performing special paradigms such as lip movement, tongue movement, swallowing and facial expression. The images were transferred to workstation for post processing and 3D reconstruction of motor homunculus. This will be useful to teach neuroanatomy and to locate primary motor areas more easily during surgery without functional deficit to the motor area.
This technique maps the physiological or metabolic consequences of altered electrical activity in the brain and can be repeated in patients and normal individuals because of its non-ionizing nature.
Echo planar imaging (EPI)
It is a fast magnetic resonance technique, which is used to sequentially acquire brain images every few seconds (TR=2000ms-TE=4000ms) during several minutes of data acquisition. Its minimum acquisition time makes it ideal for fMRI acquisition.
fMRI Paradigms
fMRI detects the difference in electrical activity of brain neural signaling by difference in magnetic property between oxyhemoglobin and deoxyhemoglobin using a special technique known as Blood Oxygen Level Dependent (BOLD) Technique (Thulborn et al. 1982). During fMRI image acquisition, the subject as asked to perform several tasks such as swallowing, tongue movement with closed lip, lip movement, facial expression and visual -blinking movement. Each of these tasks was repeated several times and was separated by rest periods. The combinations of these tasks and resting states were known as fMRI paradigms.
Blood Oxygen Level Dependent (BOLD) Technique
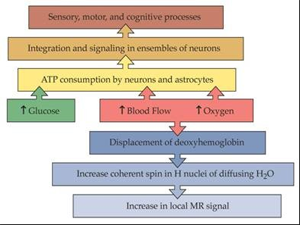
The detection of functional areas of the brain was based on blood oxygen level dependent technique which created a difference between oxy and deoxyhemoglobin in each area of the brain when special task was performed. The biophysical basis of BOLD technique in fMRI was based on paramagnetic deoxyhemoglobin and diamagnetic oxyhemoglobin in an fMRI sequence. When neurons were stimulated during a task in an MRI scanner, it lead to local increase in energy and oxygen consumption in functional areas, and the hemodynamic changes transmitted via neurovascular coupling were measured using fMRI.
Paramagnetic deoxyhemoglobin produced local field in homogeneities in a magnetic field whereas the diamagnetic oxyhemoglobin did not interfere with the magnetic field (Ogawa and Kwong 1992). When neurons were stimulated, there was an increase in local oxygen consumption that resulted in an initial decrease of oxyhemoglobin and increase in deoxyhemoglobin in the functional areas. To provide the active neurons with oxygenated blood, perfusion in capillaries and draining veins was enhanced with local oxyhemoglobin in several seconds. As a result, the initial decrease of local oxyhemoglobin was equalized and then over compensated. The progressive washout of deoxy hemoglobin by oxyhemoglobin caused a reduction in homogeneity in local field and increased BOLD signal in an f-MR image.
Study Design and Data Acquisition
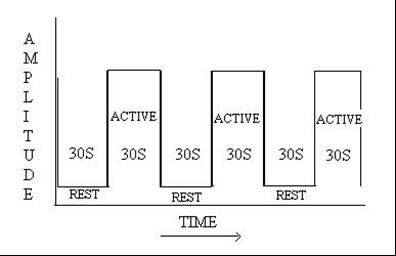
During functional imaging, the images were acquired in a few seconds at quicker rate, when a subject performs a task that shifted the brain activity between active state and rest state. This could be explained by a simple BOXCAR design. This study design was simple and the average time consumed was efficient approach for comparing brain responses in different states (active state and rest state). An alternate approach of event-related paradigm was performed in the task between active and rest states, when the scanning was in progress.
Similar five sets of images were acquired in active state (30 sec) and rest state (30 sec) each, when the scan was in progress using boxcar study design. The signal time course in each voxel of the slices and the time course of different tasks were correlated. This could identify voxels in brain that showed statistically significant changes associated with brain functions. This statistical map (z-score) was superimposed on a high resolution anatomical image by using co-registration technique for proper identification of the precise anatomic location of origin of the signal. Since the procedure was cumbersome, it was carried out in a post processing work station of Siemens.
f- MRI Mapping of Eloquent Cortex
fMRI could obtain data preoperatively and non-invasively together with high sensitivity for visualizing brain lesions. It could distinguish and define the relation between the margin of a lesion and any adjacent functionally significant brain tissues. FMRI had the potential to predict possible deficit in motor and sensory perceptual functions and also to localize the motor homunculus in relation to the lesions in the brain. The usefulness of fMRI was to select the subject for surgery, tailoring surgical resection and in predicting the postsurgical outcome.
Observations
Swallowing movement in coronal sections
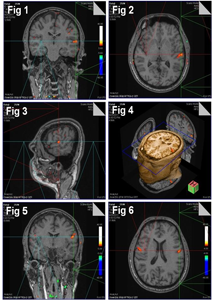
The functional activation of swallowing movement was seen at the motor area 4 on the superolateral surface of the cerebral hemisphere (Fig. 1)
The activation glow corresponded to the 3rd ventricle just above the lateral sulcus as in coronal sections. Functional glow was not seen on the right side at this level. Less functional activation specs of glow were on the superolateral surface of the cerebrum above the one mentioned on the left side. A small spec of glow was on the right side corresponding to the level of lateral ventricle.
Swallowing movement in axial sections
Similar functional activation was on the superolateral surface of the cerebral hemisphere on the motor area 4, which corresponded to 3rd ventricle as in axial section (Fig. 2). Functional glow was not on the right side at this level.
Swallowing movement in sagittal sections
Sagittal sections showed functional activation glow on the superolateral surface of motor area 4 in front of Rolandic sulcus, 5 cm above the external auditory meatus (Fig. 3). A speck of activation glow was in motor speech area 44, 45 and two specs of activation glow in the premotor area 6, 8.
3 Dimensional representation of swallowing area
3Dimensional representation of image on axial plane showed functional activation in the superolateral surface of the cerebral hemisphere on the motor area 4 with reference axial, coronal and sagittal planes in it (Fig. 4) This had been brought together to represent the swallowing area in somatomotar area 4.
Tongue movement in coronal sections
The functional activation of tongue movement was bilaterally on the superolateral surface of the cerebral hemisphere at the motor area 4, which corresponded to the level of lateral ventricle, fornix and septum pellucidum as in coronal section (Fig. 5). The activation glow was more on the left side with stoop activation on the right Side.
Tongue movement in axial sections
The functional activation of tongue movement was seen bilaterally on the superolateral surface of the cerebral hemisphere at the motor area 4, which corresponded to the level of body of lateral ventricle, fornix, and septum pellucidum as in axial section (Fig. 6).
Tongue movement in sagittal sections
Functional activation was at the motor area 4 on the superolateral surface of the cerebral hemisphere, 5 cm above the external auditory meatus (Fig. 7).
3Dimensional representation of tongue movement
3Dimensional representation of functional activation of tongue movement was in coronal section (Fig. 8), at the motor area 4 on the superolateral surface of the cerebral hemisphere with reference axial, coronal and sagittal planes. The functional activation was bilaterally at motor area 4 on the superolateral surface just above the swallowing area.
Lip movement in coronal sections
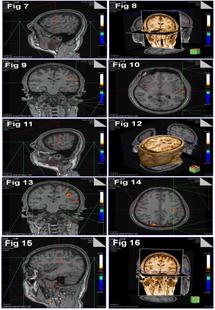
Coronal section showed the functional activation on the superolateral surface of the cerebral hemisphere, just above the lateral sulcus at the level of 3rd ventricle. There was no glow at this level on the right side. There was stoop activation on the left hemisphere above the area of functional activation at the level of lateral ventricle as in coronal sections (Fig. 9). A few specs of diminishing activity were bilaterally on the right and left hemisphere, which corresponded to each other, above the level of lateral ventricle.
Lip movement in axial sections
Functional activation of lip movement was in motor area 4 on the superolateral surface of the cerebral hemisphere in axial sections (Fig. 10).
Activation was more on the left side. There was no activational glow on the right Side at the level of 3rd ventricle as in axial section (Fig. 10). A diminishing glow was on the right side corresponding to the lateral ventricle.
Lip movement in sagittal sections
Functional activation was above the lateral sulcus. The activation lay 5 cm above the tragus (Fig. 11), which corresponded to the somatomotor area of the cortex. A few diminishing glow was on the motor area above the functional activation.
3Dimensional representation of lip movement
3 Dimentional representation of lip movement on the motor cortex was in all the 3 axial, coronal and sagittal reference planes with central axial 3D image (Fig. 12), above tongue movement. The diminishing activity was noted in sagittal and coronal reference planes.
Facial expression in coronal sections
In coronal section, functional activation was on the superolateral surface at the level of body of corpus callosum (Fig. 13). There was no glow on the right Side at this level. A few diminishing activations were on the left side above the functional area of activation. There was a speck of glow above the level of corpus callosum on the right side.
Facial expression in axial sections
Functional activation of facial expression was on the superolateral surface of the cerebral hemisphere on the left side. There was neither similar nor small glow at this level on the right side of axial section. Little activation was in front of the above said glow on the right side.
Functional activation was at the area 17, 18, 19 on the right occipital lobe corresponding to the left hemisphere. The occipital lobe showed a few diminished activations in area 17 as in axial sections (Fig. 14).
Facial expression in sagittal sections
Functional glow was on the superolateral surface of the cerebral hemisphere above the circular sulcus of insula. There was also a little functional activation above, below and behind the above mentioned glow (Fig. 15).
3Dimensional representation of facial expression
3Dimensional representation of facial expression was in the coronal image with reference axial, sagittal and coronal planes. Coronal section revealed activation on the left side with diminishing activity on the right side visual area (Fig. 16).
Discussion
Using Siemens 1.5 T, fMRI were acquired by performing special paradigms such as swallowing, tongue movement, lip movement and facial expression. The images were transferred to workstation for post processing and 3Dimensional reconstruction of motor homunculus of the face.
Swallowing movement
The functional activation of swallowing movement was at the motor area 4 on the superolateral surface of the cerebral hemisphere on the left side. The glow corresponded to the 3rd ventricle above the lateral sulcus in coronal, axial and sagittal sections. The functional glow well correlated with the electrical stimulation, which was a gold standard method. Less activated specs of functional glow were on the superolateral surface on both side cerebral hemispheres. It could have been due to slight movement of the muscles of the face.
Swallowing movement in sagittal section revealed the same findings as that of coronal and axial sections. A few specs of functional glow were on the motor speech area 44, 45 and premotor area 6 and 8. It could have been due to the coordinated movement of the swallowing area with premotor and motor speech area.
fMRI and electrical stimulation area were highly concordant (Stephani et al. 2000). These findings might enable the neurosurgeons to locate the primary motor area easily during surgery. High correspondence was between the somatotopic anatomy and function in Rolandic sulcus (Stephani et al. 2000).
Tongue movement
Functional activation of the tongue movement was bilaterally at the motor area on the superolateral surface of the cerebral hemisphere, which corresponded to the level of lateral ventricle as in coronal section (Fig. 5). The activational glow was more on the left side because of dominant hemisphere. The stoop activation in the right side could have been due to possible bilateral cortical representation, tongue being a midline organ as in axial section (Fig. 6)
In sagittal section, functional activation was at the motor area on the superolateral surface of the cerebral hemisphere, 5cm above the external auditory meatus (Fig. 7).
3Dimensional observations showed bilateral representation of tongue movement clearly in all the 3 reference planes (Fig. 8).
Anatomical central location of global maximum intensity for each individual might vary, that depended on individuals task complexity, paradigm design, data analysis techniques or a combination of them, which formed the basis of MR imaging and the functions were related to anatomical foci (Vincent et al. 2006).
Lip movement
Functional activation was on the superolateral surface of the left dominant cerebral hemisphere, just above the lateral sulcus at the level of 3rd ventricle as in coronal section (Fig. 9). Stoop activation was above the area on the left side. This could have been due to group activity of the muscles of facial expression.
A few specs of diminishing activity bilaterally corresponded to each other on the right and left hemispheres above the level of lateral ventricle. This showed the bilateral cortical representation of the upper part of the face in the facial nerve nucleus. It also correlated with clinical cases of supranuclear injury of facial nerve. Only lower half of the face was paralyzed and upper half of the face was spared.
In addition to functional glow at the motor area in sagittal section lip movement, a few diminishing glow was above and in front of lip area It could have been due to group action of the muscle of facial expression.
Facial expression:
Functional activation of facial expression was on the superolateral surface of the left hemisphere at the level of trunk of corpus callosum (Fig. 13). No glow was on the left side. There were a few diminishing glows on both the hemisphere at this level. The diminishing glow was bilateral and indicated bilateral cortical representation of upper half of the face. Functional activation on the right side also indicated the bilateral cortical representation.
Functional activation was on the right calcarine sulcus area 17 & 18 on the left hemisphere and a few diminishing activity in area 17 of right hemisphere in axial section (Fig. 14). This could have been due to the blink reflex during facial paradigm.
Functional glow was also on the superolateral surface above the circular sulcus of insula in sagittal section. There was also a little functional activation above, below and behind the functional area of the left hemisphere. This could have been due to group activity of the muscles. All this diminishing activity was only in the pre-Rolandic area. This could have been due to stimulation of cerebral cortex in response to cerebral activity.
High functioning individual with autistic disorder differed from normal individual in the activity of cerebellum, limbic and temporal lobe corical region of the brain, when processing facial expression (Critchly et al. 2002). They did not activate the cortical face area when explicitly (consciously) or the lt. amygdala or the lt. cerebellum, when implicitly (unconsciously) processing the emotional facial expression. This could be due to Neuro developmental in origin.
Using non-invasive fMRI, it was possible to localize functional brain activation in normal individual, with an accuracy of millimeters and temporal resolution of seconds. Though numerous technical challenges remained fMRI was increasingly becoming a key method for understanding the topographical organization of the human brain. Combination of the navigation system and fMRI was useful for preoperative design of the surgical strategy and tumor orientation during the operation, enabling aggressive surgery to be performed without functional deficit. (Morioka et al 2001).
fMRIi was useful during resection of tumor in the motor area and help the surgeon to avoid or minimize post operative functional deficit and was crucial preoperative decision step for 90% of brain tumors (Klink et al. 2012).
fMRI task might replace the invasive gold standard of electrical stimulation (Brockway et al 2004).
Conclusions
The amount of motor cortex involved was directly proportional to the density of innervations and not the area of body surface. fMRI could be used to learn functional neuroanatomy of brain by constructing motor homunculus. It could also be used to assess the motor outcome of patient with various neurological disorders. The diagnostic information of fMRI permitted functional preservation and safe treatment. Mapping motor homunculus – swallowing, tongue movement with closed lip, lip movement, facial expression using fMRI could identify the eloquent cortex and predict post operative deficit of specific functions during the pre surgical works. These findings might enable the neurosurgeons to locate primary motor area more easily during surgery. fMRI was a sensitive and specific method for mapping language and motor functions. BOLD technique was non–invasive and alternative to invasive stimulatory method. fMRI and DTI were used to assist in preservation of structure and functions of motor system, which promised to decrease the patient’s morbidity and to broaden the clinical applications of functional imaging. Local relationship of cerebral tumors and sub-cortical fiber tracts could be defined. FMRI passive paradigm was an alternative and complementary to active movement task in patient population. FMRI imaging enabled the selection of more aggressive therapeutic approach that might otherwise be considered as functional risks. In certain patients surgical time might be shortened, the extent of resection was increased and craniotomy size was decreased. Repeated electrical stimulation might weaken the neuronal activity. This disadvantage was overcome by non-invasive BOLD technique of fMRI. Though numerous technical chall-enges remained, fMRI was increasingly becoming a key method for understanding the topographical organization of human brain. This non-invasive fMRI might replace the invasive gold standard electrical stimulatory method.
Acknowledgement
Dr.C. Amarnath, Dr. Philson J Mukkada, Dr. Selvakumar Vettivel, Mr. Arun.
References
Sawada K, Sasaki T, Maeno M, Yanashima K, Magatani K (2007) The BOLD signal response for fluctuating stimulation images. Conf Proc IEEE Eng Med Biol Soc, 3396-3399.
Golder W (2002) Functional magnetic resonance imaging – basics and applications in oncology. Onkologie, 25: 28-31.
Morioka J, Nishizaki T, Tokumaru T, Uesugi S, Yamashita K, Ito H, Suzuki M (2001) Functional magnetic resonance imaging-controlled neuronavigator-guided brainsurgery: a case report. J Clin Neurosci, 8: 283-285.
Byrd KE, Romito LM, Dzemidzic M, Wong D, Talavage TM (2009) fMRI study of brain activity elicited by oral parafunctional movements. J Oral Rehabil, 36: 346-361.
Vincent DJ, Bloomer CJ, Hinson VK, Bergmann KJ (2006) The range of motor activation in the normal human cortex using bold FMRI. Brain Topogr, 18: 273-280. 3.
Krings T, Reinges MH, Thiex R, Gilsbach JM, Thron A (2001) Functional and diffusion-weighted magnetic resonance images of space-occupying lesions affecting the motor system: imaging the motor cortex and pyramidal tracts. J Neurosurg, 95: 816-824.
Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy DG (2000) The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain, 123: 2203-2212.
Cohen MS, Bookheimer SY (1994) Localization of brain function using magnetic resonance imaging. Trends Neurosci, 17: 268-277.
DeYoe EA, Bandettini P, Neitz J, Miller D, Winans P (1994) Functional magnetic resonance imaging (FMRI) of the human brain. J Neurosci Method, 54: 171-187.
Cohen MS, Susan Y, Bookheimer (1994) Localization of brain function using magnetic resonance imaging. Trends in Neurosci, 17: 268–277.
Lehéricy S, Duffau H, Cornu P, Capelle L et al., (2000) Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg, 92: 589-598.
Suzanne T, Alexandra G (2007) Functional Brain Mapping and Its Applications to Neurosurgery. Neurosurgery, 60: 185-202 .
Kocak M, Ulmer JL, Ugurel MS, Gaggl W, Prost RW (2009) Motor Homunculus: Passive Mapping in Healthy Volunteers by Using Functional MR Imaging—Initial Results. Radiology, 251: 485-492.
Petrella JR, Shah LM, Harris KM, Allen H, et al., (2006) Preoperative Functional MR Imaging Localization of Language and Motor Areas: Effect on Therapeutic Decision Making in Patients with Potentially Resectable Brain Tumors. Radiology, 240: 793-802.
Bizzi A, Blasi V, Falini A, Ferroli P, et al., (2008) Presurgical Functional MR Imaging of Language and Motor Functions: Validation with Intraoperative Electrocortical Mapping. Radiology, 248: 579-589.¬
Rajaram and Govindarajan – Crossed Fused Renal Ectopia