International Journal of Anatomical Sciences 2014, 5(1):17-25
Research Article
Delayed Transplantation of Human Amniotic Epithelial Cells in Transient Focal Cerebral Ischemia Induced Rat Brain Can Survive and Ameliorate Functional Recovery
R.Ramesh Kumar, P.Ravisankar, R.Muthusamy
Department of Anatomy, Dr.A.L.M.PGIBMS, University of Madras, Taramani campus, Chennai – 600 113.
Department of Anatomy, Melmaruvathur Adhiparasakthi Institute of Medical sciences and Research, Melmaruvathur – 603 319.
Department of Anatomy, Melmaruvathur Adhiparasakthi Institute of Medical sciences and Research, Melmaruvathur – 603 319.
Key words: human amniotic epithelial cells, cerebral ischemia, transplantation, motor function
Abstract: Cerebral ischemia sets off a series of interrelated events resulting in neuronal injury and functional deficits. Human amniotic epithelial cells found to be immunologically naïve and proved to be an effective substitute to adult, embryonic and fetal stem cells for neurological diseases, when transplanted in different animal species. In this study cerebral ischemic reperfusion injury was created in the wistar albino rats and after 7 day of reperfusion HAE cells was transplanted in the ischemic area and the HAE cell survival and motor behavioral outcome was assessed through neurobehavioral tests. HAE cell was found to survive and migrate in the ischemic brain till 30 days after transplantation and also they showed improvement in rotorod test performance and reduction in the foot fault error in the narrow runway task. The outcome of this study confirms that HAE cell is a potent substitute for neural cells and can survive in the adverse environment of ischemic brain ameliorating functional recovery.
Brain tissues are more susceptible to any small alteration in their normal environment and produce drastic changes in the general body systems. Amongst the neuronal diseases or disorders, stroke or the cerebrovascular disorder stands the world’s third leading dreadful disease and a leading cause for long-term morbidity among the world population (Lo et al., 2003). Increase tobacco use, diet leading to overweight and obesity, high blood pressure (BP), high blood cholesterol and less physical activity are the major risk factor for stroke. Stroke is 5 to 10 percent higher in the countries such as Russia, India, China, Pakistan and Brazil when compared to UK and USA (Gorelic, 2009).
During cerebral ischemia as the supply of oxygen and glucose to the brain tissue is lost the production of high energy phosphate compounds such as adenosine triphosphate (ATP) falls, leading to failure of energy dependent processes necessary for neural cell survival. This sets off a series of interrelated events that result in neuronal injury. These include the failure of mitochondrial function, electrolyte imbalances in brain cells, increase in the intracellular calcium level, release of excitatory neurotransmitters, production of oxygen free radicals and other reactive oxygen species.
In the fully matured brain, the striking disadvantage is inability of neurons to regenerate, so when there is a permanent loss of neuron, it is not substituted by a new generation of similar cells. In this instance the transplantation of embryonic stem cells had made a tremendous benefit in substituting the neuronal loss and regaining the function loss. But they have their own limitation on ethical issues and species specificity. The transplantation of adult, embryonic and fetal stem cells has shown promising development in improving the behavioral deficits in experimental models of neurological diseases. Although ethical and political issues have limited the use of fetal stem cells; cell resources and rejection have restrained the use of adult stem cells.
The human placental derived amniotic epithelial cells isolated from the placenta after delivery has been proved as a potential graft source which express markers for both neural and glial cells and when transplanted show no immune rejection (Sakuragawa, et al., 1996,1997). Further studies on Human amniotic epithelial (HAE) cells have shown that they has the potentiality to synthesize and secrete various neurotransmitters such as epinephrine, nor-epinephrine, dopamine (Elwan, 1998) acetyl-choline (Elwan and Sakuragawa., 1997) and erythropoietin (Terada et al., 2000). HAE cells was found to lack histocompatitbilty complex antigen (Terada et al.,2000), secrete neurotrophic factors such as brain-derived neurotrophic factor, neurotrophin-3, and nerve growth factor (Uchida et al., 2000). They do not express telomerase and found to be non-tumerogenic upon transplantation (Miki et al., 2005). Transplantation of HAE cells into rat midbrain prevented the degeneration of nigrostriatal dopamine neurons after injection of 6-hydroxydopamine (Kakishita et al., 2003). When HAE cells transplanted in to the monkey spinal cord after injury, it reduced the formation of glial scar and attracted the growth of new collateral sprouting and improved the hind limb motor function (Sankar and Muthusamy. 2003). Moreover the HAE cells are useful and noncontroversial source of stem cells for all transplantation and regenerative medicine (Miki.T et al., 2005) and do not invoke any religious, ethical or legal issues like human fetal cortical tissue.
Considering the above facts, This study was designed to evaluate the efficacy of HAE cells by delayed transplantation in the ischemic brain of wistar albino rat after transient focal cerebral ischemia induced by middle cerebral artery occlusion.
Materials and Methods
Animals and Experimental design.
Healthy adult male wistar albino rats weighing about 250 to 300 grams of body weight were used for this study. Rats were housed in polypropylene cages with paddy husk, maintained under standard atmospheric condition of 12 hour light and dark cycle at a constant temperature of 250± 30C and 30% to 60 % humidity. Rats were fed with standard rat pellet diet and water ad libitum. This entire study was approved by the Institutional Animal Ethical committee. All efforts were made to minimize both the number of rats used for this study and unwanted stress or discomfort to the rats during experimental procedures.
Rats were grouped as (i) Sham (Rats underwent the surgical procedure except Middle cerebral artery occlusion(MCAo)), (ii) Lesion (Rats exposed to 2 hours of MCA occlusion and reperfusion) and (iii) HAE Transplantation group (Transplantation of HAE cells on day 7 following 2hrs of MCAo and reperfusion).All the rats were assessed for motor behavioral function and were sacrificed for histological assessment on day7 and 30 after transplantation.
Induction of transient focal cerebral ischemia.
To create transient focal cerebral ischemia in wistar albino rats, the surgical procedure suggested by Longa, et al (1989), by intraluminal filament occlusion of Middle Cerebral Artery was adopted. Rats were deprived of food but not water for 8 hours prior to surgery and anesthetized with intraperitoneal administration of Thiopental sodium (40mg/kg body weight) with a pre anesthetic intramuscular administration of Atropine (0.5 mg /kg body weight,). The ventral aspect of the neck was shaved thoroughly and cleaned with antiseptic solution. Then a ventral midline incision was made in the neck and the underlying connective tissue layers were incised and separated in layers. The right common carotid arteries (CCA), internal carotid artery (ICA), external carotid artery (ECA) were isolated from the surrounding nerves plexus and connective tissues. The branches of ECA were isolated and cauterized using micro diathermy bipolar electrodes (Martin – Germany). Distal segment of the ECA was ligated using 4 0 silk suture and a loose not was made around the proximal segment of ECA. The CCA and ICA were temporarily occluded using micro vascular clips (FST ,USA) and a small nick was made in the ECA and the pre prepared 4 0 nylon monofilament (Belayev, et al., 1996) was introduced in to the ECA and the lose knot near the proximal segment was tightened. The distal segment of ECA was detached from the proximal segment and the micro vascular clips from CCA and ICA were removed. Then the Monofilament was redirected in to the ICA till a resistance is felt (Approximately 18mm – 19mm length). The surgical area was closed and sutured in layers, retaining the monofilament in position. After 2hrs of occlusion the monofilament was withdrawn and reperfusion was established.
Human Amniotic Epithelial (HAE) cells isolation and transplantation
HAE cells isolation was done as described by Sakuragawa et al., (1996). The connective tissue from the amniotic membrane was scrubbed and removed. The membrane was than cleaned with phosphate buffered saline (PBS) thoroughly and trypsinised in 0.125% trypsin (Hi-media) in PBS for 3 changes of 20 minutes each. The pellets so obtained after each treatment were re-suspended in PBS and pooled together and washed in fresh PBS for 3 times. The HAE cells so obtained were suspended in Dulbaco’s Modified Eagle Medium (DMEM) with HEPES (Hydroxy ethyl piperazine sulphonic acid) buffer (Himedia, India) and supplemented with 10% fetal bovine serum. The HAE cells were then maintained in a CO2 incubator in a humidified atmosphere of 5% CO2 in air at 37º C. The culture was maintained till the host animal was ready for transplantation. HAE cells viability was assessed before transplantation through Trypan blue exclusion method and cells was used for transplantation only when the viability was more than 85%.
After 7 days of reperfusion the group iii rats were anesthetized with Ketamine, Xylazine at a dose of 100 mg/kg body weight and 10mg/kg body weight respectively. HAE cells was injected in to the ischemic area of rat brain through small burr holes with the aid of stereotaxic apparatus (Inco, India) based on the following coordinates (i) Antero-posterior (AP) = 1.08 , Right lateral (RL) = 3 mm, and Dorsoventral (DV) = 6 mm from the dural surface; (ii) AP = -1.08 mm, RL = 3 mm, and DV = 4mm from the dural surface. Using a 10μl Hamilton syringe fitted to the manupulator of the stereotaxic apparatus 2μl of cell suspension (1 X 104 cells/μl) was slowly injected into each site of the brain as per the above mentioned coordinates. After transplantion, the needle was left in place for 10 minutes and then withdrawn slowly. The surgical incision was closed in layers. The rats were left undisturbed for two hours and then they were taken for post-operative management.,
Neurobehavioral assessments.
All the rats were assessed for their motor behavior through the following tests by experimentally blind investigators.
Rotarod Test (Chen, et al., 2001)
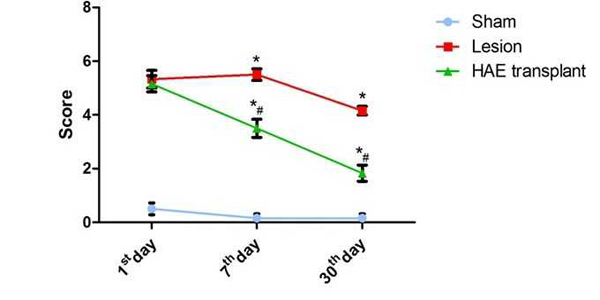
In this test the rats were placed on the rotarod cylinder and the time the animal remained in the rotarod was measured at the constant speed of 20 rpm for maximum of 5 minutes. The trial will be ended if the animal falls off the cylinder before 5 minutes or spuns around the beam for more than 2 consecutive rotations without attempting to walk. The rats trained priorly for this test alone were included in the experimental groups. After lesion as per the scheduled groups and period of study, three trials were conducted and the mean value was recorded. A score of 0,1,2,3,4,5,6 was assigned to the performance of the rats as tabulated below.
| Animal status | Score |
| Balances with steady posture | 0 |
| Balances the beam ,but grasps the side of the beam | 1 |
| Hugs beam and 1 limb falls off the beam | 2 |
| Hugs beam and 2 limb falls off the beam or spins on beam (>60s) | 3 |
| Attempts to balance the beam ,but falls off(>40s) | 4 |
| Attempts to balance the beam ,but falls off(>20s) | 5 |
| Attempts to balance the beam ,but falls off(<20s) | 6 |
Narrow Runway Test (Kunkel, et al., 1993)
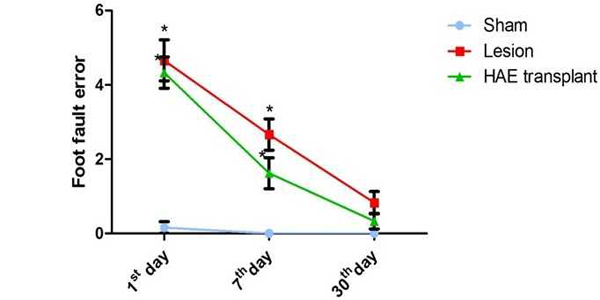
The narrow runway is a wooden beam of 2.5 cm wide, 100 cm length and 10 cm height. Rats were made to run on the beam and the duration to cross the beam, number of steps and forelimb errors were measured during the evaluation. An error was counted when the animal foot misses the beam and slips over the side. Before lesion rats were trained for 7days and the mean scores were recorded for three trials. After lesion as per scheduled groups and study period rats were consecutively assessed for three trials and the mean value was recorded.
Histological and immunohistochemical analysis.
The rats were euthanized using thiopental sodium at a dose of 80mg/Kg body weight intrperitoneally on scheduled study periods, rats were transcardially perfused with 0.1 M Phosphate buffered saline (PBS) and then with 4% paraformaldehyde in PBS and the rats were decapitated and the brain was removed and stored in 4% paraformaldhyde in PBS at 4oC. Histological staining was performed through paraffin processing as described previously. Briefly, sections were cut in the coronal plane at 10 micron thickness and mounted on slides. Sections were deparaffinated and rehydrated. Sections were stained with hematoxylin–eosin (H & E) for morphological analysis.
To confirm the survival of HAE cells in the group iii rats the brain block selected from the lesion site was processed for immunohistochemical staining. The brain tissue was impregnated in 25% sucrose in PBS till the section sinks completely in to the solution. Then the brain tissue was embedded with Bright Cryo-M-Bed (Bright instrument company Ltd, England) and 20 μm thick section were taken in a cryostat (Leica CM 1510S, Germany) for immunohistochemical staining. The tissue sections obtained were stained for Human cell marker with Mouse anti Human nuclei monoclonal antibody(Anti HuNu, Chemicon International Inc) and Rabbit anti mouse IgG conjugated with TRITC was used as the secondary antibody. The brain section was observed under Nikon labophot episcopic fluorescence attached microscope. For TRITC Nikon G2A; Excitation filter 510 ~ 560; Barrier filter 590.
Statistical Analysis
All the data were expressed as Mean ± SEM and was analyzed by two way analysis of variance (ANOVA) followed by Bonferroni test and p values ≤ 0.05 were considered as statistically significant.
Observation
Human Amniotic Epithelial (HAE) cell transplantation and animal behavior:
In the rotarod test (FIG. 1) the score of HAE cell transplanted rats on day1 after transplantation was not significant when compared to lesion rats. But performance of the HAE cell transplanted rats on day 7 and 30 there was a significant improvement in the rotarod performance score when compared with Lesion.
In the narrow runway test (FIG.2), the HAE cells transplanted rats showed a marginal reduction in the foot fault errors on all the study periods when compared to the lesion rats; but the values were not statistically significant.
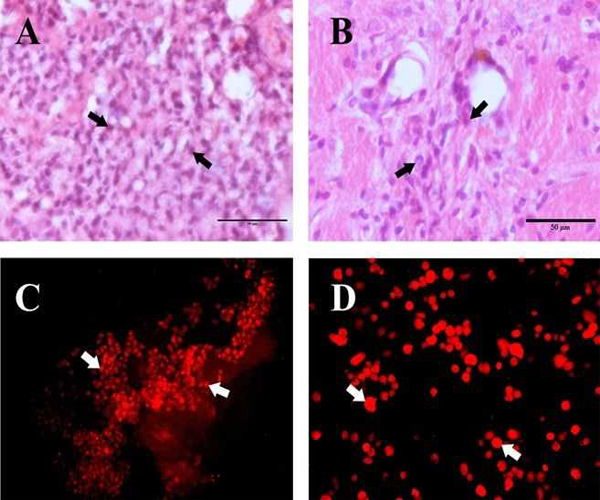
The HAE cells transplanted on day 7 after MCAo in the ischemic cerebral hemisphere of wistar albino rats was found to survive, migrate and integrate with the host cells. On day 7 after transplantation HAE cells were observed in cluster at the site of injection in the ischemic rat brain. In the H&E stained section of the transplanted Ischemic cerebral hemisphere, HAE cells appear in clusters with shrunken and basophilic cytoplasm (FIG 3A). A few cell groups were found to be surrounded by the macrophages already existing in the ischemic area. Further the positivity of Anti Human nuclei (HNu) antibody (FIG.3C) confirms the survival of HAE cells at the transplanted site on day 7 after transplantation.
On day 30 after transplantation, the overall transplanted HAE cell population was found to be reduced (FIG.3B). The HAE cell morphology was altered from round to oval shape. The cells were found to be enlarged and migrated towards the ischemic area (FIG.3B). A few cells showed a synaptic network with the existing viable neurons in the ischemic hemisphere. The Anti HNu antibody positive cells were found to be dispersed in the ischemic zone confirms the survival and migration of HAE cells on day 30 after transplantation (FIG.3D).
Fate of Human Amniotic epithelial cells transplanted in the ischemic cerebral hemisphere:
Discussion
There is no effective means for replacing brain cells and restoring its function after permanent loss due to neural disorders. Fetal tissue transplantation made a remarkable development in substituting damaged brain tissues and improving the functional deficits. Various experimental studies have been conducted to evaluate the status of embryonic and foetal neural tissue transplantation in the ischemia induced neuronal damage has shown promising development in improving the behavioral deficits in experimental models of neurological diseases (Hadani et al., 1992; Koide et al., 1993; Athara et al., 1994). But ethical and political issues have limited the use of fetal stem cells, cell resources and rejection have restrained the use of adult stem cells. Human amniotic epithelial (HAE) cells , derived from the placenta after parturition was considered to be the alternative source for fetal and embryonic stem cells in substituting the neural loss due to various neurological disorders. Human amniotic epithelial cells are immunologically naïve (Adinolfi et al., 1982). Invitro and invivo studies on HAE cells has shown remarkable outcome by expressing neural and glial markers and also found to express certain neurotrophic factors and neurotransimitters, when transplanted in various neurological disease animal models (Sakuragawa. et al.,1996; Terada et al., 2000; Kakishita et al., 2003)
In this study, rats that were intracerebrally injected with HAE cell on 7th day after MCAo, showed improved motor performance assessed through rotarod stability test and narrow runway for foot fault error test till 30 days after transplantation. A similar study on HAE cell transplantation in MCAo rats and assessment for 2 weeks was shown to enhance motor function through different neurobehavioral tests (Liu et al., 2008)
In the present study on day 1, the HAE cell transplanted animals did not show any difference in performance of the behavior task, this may be due to non-integration or delayed adaptation of HAE cells with the host brain. This study also document the role of HAE cells in improving the motor function of rats after focal cerebral ischemia through morphological modification and integration with the surviving host neuronal cell population. We were able to detect some HAE cells dispersed from the site of injection to the peripheral infarct zone on day 7 and 30 after transplantation. This demonstrates that intracerebral delivery of HAE cells promotes survival and potential migration into the rat brain. In earlier studies the presence of Human umbilical cord blood cell in the ischemic rat brain was located by the mouse anti human nuclei antibody (Chen, et al., 2001). Similarly in this study the mouse anti human nuclei antibody, a human cell marker made a significant contribution to locate the dispersed HAE cells in the ischemic brain.
Furthermore, HAE cells increased the ability to improve the behavioral recovery of ischemic rats and reduced the infarct size rapidly and stably. These results support the assertion that HAE cells may be a cell resource for cell therapy and useful vehicles for gene therapy in neurodegenerative disease ( Liu.,et al 2008 ).
After ischemia a vast population of neural glial cells undergoes permanent damage and further evokes inflammatory reactions and apoptotic cell death, worsening the functional loss and increasing the infarct area progressively. The known neurotrophic factors BDNF, NT- 3 expressed by HAE cells (Uchida, et al., 2000) and other growth factors secreted by HAE cells may partially explain their therapeutic mechanism, but the mechanism of functional recovery after transplantation of HAE cells is still questioned and need to be answered through further investigations. This study has given a primordial document to show the survival of HAE cells till 30 days after transplantation in the ischemic brain after delayed transplantation.
Conclusion
In conclusion the HAE cells as a substitute to embryonic stem cells can be an effective stem cell for neurodegenerative disorders, but require further validation on its long term benefits and status in animal models for successful application in clinical trials.
References
Adinolfi M, Akle CA, McColl I, Fensom AH, Tansley L, Connolly P, Hsi BL, Faulk WP, Travers P, Bodmer WF (1982) Expression of HLA antigens, beta 2-microglobulin and enzymes by human amniotic epithelial cells. Nature, 295: 325 – 327.
Athara N, Mizukawa K, Koide K, Mabe H, Hishino H (1994) Striatal grafts in infarct striatopallidum increase GABA release, reorganize GABA receptor and improve water maze learning in rat. Brain research, 33:483 – 488.
Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg ME (1996) Middle cerebral occlusion in the rat by intraluminal suture. Stroke, 27: 1616-1623.
Chen J, Samberg PR, Yi Li Wang L, Mei Lu, Willing AE, Ramos JS, Choop M (2001) Intravenous administration of Human Umbilical Cord Blood Reduces Behavioural Deficits After Stroke in Rats. Stroke, 32: 2682-2688.
Elwan MA, Sakuragawa N (1997) Evidence for the synthesis and release of catecholamine by human amniotic epithelial cells. Neuro report, 10: 3435-3438
Gorelick PB (2009) Burden of stroke and risk factors, Bornstein NM (Ed): Stroke. Basel, Karger, pp 9-23.
Hadani M, FreemanT, Munisiff A, Young W, Flamm E ( 1992) Foetal cortical cells survive in focal cerebral infarct after permanent occlusion of the middle cerebral artery in rats. J.Neurotrauma, 9: 107 -112.
Kakishita K, Elwan MA, Nakao N, Itakura T, Sakuragawa N (2000) Human amniotic epithelial cells produce dopamine and survive after implantation in to the striatum of a rat model of Parkinson’s Disease; A potential source of donor for transplantation therapy. Experimental Neurology, 165: 27 – 34.
Kakishita K, Nakoda N, Sakuragawa N, Itakura T (2003) Implantation of human amniotic epithelial cells prevents the degeneration of nigral dopamine neurons in the rats with 6- hydroxydopamine lesion. Brain Research 980: 48 – 56.
Koide K, Hashitani T, Aihaa N, Mabe H, Nishino H (1993) Improvement of passive avoidance task after grafting of fetal striatal cell suspensions in ischemic striatum in the rat. Restorative Neurology and Neuroscience, 5:205-214.
Koizumi J, Yoshida Y, Nakazawa T, Ooneda G (1986) Experimental studies of ischemic brain edema. A new experimental model of cerebral embolism in rats in which recirculation can be introduced in the ischemic area. Japanese Journal of stroke, 8: 1 – 8.
Kunkel-Bagden E, Dai HN, Bregman BS (1993)Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Experimental Neurology, 119: 153-164.
Lo EH, Dalkara T, Moskowitz M (2003) Mechanisms, challenges and opportunities in stroke, Nature Reviews – Neuroscience 4: 399–415.
Longa EZ, Weinstein RP, Carlson S, Cummins R (1993) Reversible Middle Cerebral Artery Occlusion without Craniectomy in Rats. Stroke 20:84-91.
Liu T, Wu J, Huang Q, Hou y, Jiang Z, Zang S, Guo L (2008) Human Amniotic epithelial cells ameliorate behavioural dysfunction and reduced infarct size in the rat middle cerebral artery occlusion model, Shock, 29: 603-611.
Miki T, Lehmann T, Cai H, Stolz DB, Strom SC (2005) Stem cell characteristics of amniotic epithelial cells. Stem cells, 23: 1549-1559.
Paxinos G, Watson C (2006) The Rat Brain in Stereotaxic Coordinates, 6th edition. Elsevier Academy press, Amsterdam.
Sakuragawa N, Misawa H, Ohsugi K, Kakishita K, Ishii T, Thangavel R (1997) Evidence for active acetylcholine metabolism in human amniotic epithelial cells: applicable to intracerebral allografting for neurologic disease. Neuroscience Letters, 232: 53-56.
Sakuragawa N, Elwan MA, Uchida S, Fuji T, Kawashima K(2001) Non- Neuronal neurotransmitters and neurotropic factors in amniotic epithelial cells expression and function in human and monkey. Japanese Journal of Pharmacology, 85: 20- 23
Sakuragawa N, Thangavel R, Mizuguchi M, Hirasawa M, Kamo I (1996) Expression of markers for both neural and glial cells in human amniotic epithelial cells. Neuroscience letters, 209: 9-12.
Sankar V, Muthusamy R (2003) Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience, 118: 11-17.
Terada S, Matsuura K, Enosawa S, Miki M, Hoshika A, Suzuki S, Sakuragawa N (2000) Inducing proliferation of Human Amniotic Epithelial Cells for cell therapy. Cell Transplantation, 9: 701-704.
Uchida S, Inanga Y, Kobayashi M, Hurukawa S, Araie M, Sakuragawa N (2000) Neurotrophic function of conditioned medium from human amniotic epithelial cells. Journal of Neuroscience Research, 62; 585 – 590.