International Journal of Anatomical Sciences 2014, 5(1):26-33
Research Article
Effect of Ethanol Exposure on Heart Development in Zebra Fish (Danio rerio) Embryos
Muhammed Ibrahim, Anandan Balakrishnan, Seppan Prakash, Hyun-Jeong Lee
Department of Anatomy, Dr ALM PG IBMS, University of Madras, Chennai, India.
Department of Genetics, Dr ALM PG IBMS, University of Madras, Chennai, India.
Division of Animal Genomics and Bioinformatics, National Institute of Animal Science, Rural Development, Administration, #564 Omockchun-dong, Suwon, 441-706, Republic of Korea.
Key words: Ethanol, zebrafish, Heart, Development, Fetal Alcohol Syndrome
Abstract: The objective of the present study is to evaluate the effect of ethanol on developing zebra fish heart. Male and female fishes are allowed to breed normally and the fertilized eggs were collected and it was exposed to 3 % of ethanol (EtOH) for one hour once in 24 hours for 96 hours. The embryos were subjected to various analyses related to heart development like external morphology, heart morphology, heart rate, heart looping formation, heart length and ventricular stand still. The data showed significant alteration in the length of whole embryo or larva, heart length and heart rate of EtOH exposed embryo when compared to control. The looping also altered like string-like or straight tube appearance, with the ventricle located distinctly anterior to the atrium. Further, the ventricle appeared smaller than normal, the atrium is elongated, and both chambers having a narrow width. The incidence of ventricular standstill and valvular regurgitation is occurring in EtOH treated embryo when compared to control. Taken together, the steady decrease in heart size coupled with the severe defects in ventricular function, failure of cardiac looping formation, heart rate, ventricular standstill and retrograde blood flow would be expected to have a substantial impact on the ability of zebra fish to circulate blood. From the present study it is concluded that EtOH exposure during development results in structural and functional impairment in heart that mimic malformations that occur in patients with fetal alcohol syndrome.
Prenatal alcohol exposure has been associated with inauspicious effects like developmental delays and infant mortality, leading known cause of mental retardation in human fetuses (Abel and Sokol 1991). Gestational alcohol exposure has been shown to contribute symptoms of fetal alcohol syndrome (FAS) (Jones et al., 1973). The FAS produce lots of alteration like growth retardation, facial abnormalities, sensory deficits, impaired fine motor skills, and learning deficits, including mental retardation (Stratton et al., 1996). Previously it was believed that FAS was the result of alcohol abuse, but it is now believed that smaller doses of alcohol also can have alcohol-related birth defects (ARBD) or alcohol-related neurodevelopmental disorder (ARND) (Stratton et al., 1996). Recently, the zebra fish (Danio rerio) has become an important animal model in developmental biology and neuroscience (Bilotta et al., 2001). Also, zebra fish have transparent eggs and the embryos are semi-transparent. Thus, the stage of embryonic development of an individual embryo can be determined without interfering with development. Several studies have shown that EtOH exposure can affect zebra fish embryonic development. Bilotta et al., (2004) had reported that the EtOH exposure produces cyclopia (fusion of the two eyes). Then also reported that EtOH exposure impair the development of nervous system and behavioral deficit in zebra fish embryo (Cole et al., 2012). Carvan et al., (2004) also reported that EtOH exposure alters the neurobehavioral and skeletal morphogenesis. Another study also documented that it produces severe body malformations (Baumann and Sander, 1984). Daft et al., (1986) had reported that gestational acute EtOH exposure produced abnormal heart and great vessel development in mice. Fetal alcohol spectrum disorder (FASD) describes a range of birth defects, including various congenital heart defects (CHDs). However the exact mechanisms of FASD-associated CHDs are not fully understood. The objective of the present study to analyze the effect of EtOH on developing heart using zebrafish embryos as in-vivo experimental model
Materials and Methods
Zebrafish husbandry and embryo collection
Adult zebrafish (Daniorerio) were purchased from a local authenticated supplier and housed. Zebra fish were reared in 2.0 l polycarbonate tanks on a recirculating system in which the water was maintained at 28±1 °C and at pH of 7.0±0.2. The fish were fed twice daily. Eggs were obtained by random pairwise mating of zebra fish. Three adult males and four females were placed together in small breeding tanks the evening before eggs were required. The breeding tanks (L 26 cm; H 12.5 cm; W 20 cm) had mesh egg traps to prevent the eggs from being eaten.
The eggs were harvested the following morning and transferred into 92 mm plastic Petri dishes (50 eggs per dish) containing 40 ml fresh embryo buffer. Eggs were washed four times to remove debris, unfertilized, unhealthy and dead embryos under a dissecting microscope. At 3.5 hours, post fertilization (hpf), embryos were again screened and any further dead and unhealthy embryos were removed. Throughout all procedures, the embryos and the solutions were kept at 28.5°C, in the incubator under a light cycle of 14 h light: 10 h dark (lights on at 8 h). Embryo buffer was refreshed every 24 h. Normal dividing and spherical embryos at the 256 cell stage (2.5 hpf) through the oblong stage (3.7 hpf) were selected and utilized for all of the studies described. Embryos were staged using the pectoral fin, yolk sac, anal pore, and swim bladder as indicators of developmental stage.
Ethanol exposure
The washed forty embryos in triplicates at 3.7 hpf stage were used for the present study. The embryo was exposed to 3 % of EtOH for 1 hour at 28.5°C. After the incubation the embryos were washed 3 – 4 time with a fresh embryo buffer. Embryos were kept in an incubator at 28.5°C, with a refreshment of the buffer once in a day for 96hpf. Every 24 hpf the embryo were observed and analyzed for morphological ad, developmental changes of heart.
Morphological analysis
The EtOH exposed embryos were subjected to morphological analysis like viability of the embryo, length of the embryo, status of pericardial sac and heart shape. The embryo was placed in a lateral position and was analyzed qualitatively and quantitatively.
Zebrafish embryos were positioned in 3% methylcellulose and kept on the lateral side to get a better view of functioning heart (Duan et al., 2013). Ventricles beats are counted in 20s periods. At least thirty measurements were taken and their average was used in statistical analysis. In these experiments the heat rates for EtOH exposed embryo were measured in the same observation session under identical conditions like control.
Estimation of SV-BA distance
Changes in heart morphology caused by EtOH exposure were measured (Mehta et al., 2008) by positioning embryos in 3% methyl cellulose to allow capturing of lateral view images and measurement of the distance between the sinus venosus (SV) and bulbus arteriosus (BA) regions of the heart. The image was captured and was subjected to distant measurement using stage and occulo meter scale.
Incidence of ventricular standstill.
Zebra fish larvae were assessed for the incidence of ventricular standstill at 96 hpf as described previously (Antkiewicz et al., 2005). The embryo or larvae were observed for larvae persistent lack of visible ventricular contraction and scored as exhibiting the ventricular standstill. At least three separate experiments were performed (n =40 larvae per each EtOH exposed embryo per experiment), and the average percentage of larvae exhibiting ventricular standstill for each group was calculated.
Heart looping formation
To assess the angle of looping between the atrium and ventricle, the developing heart will be studied through recordings of the beating heart and image will be analyzed with manual method by analyzing the orientation of each chamber.
Peripheral blood flow
Video images of blood flow in the posterior inter segmental vein in the trunk of embryos will be captured and the number of red blood cells passing a chosen landmark in the posterior inter-segmental vein during 10 s will be counted.
Statistical analysis
The significant difference between the mean value of control and experimental groups were analyzed according to the method of Zaire (1974). All the data were subjected to Student‘t’ test and data showing p value < 0.05 was considered as statistically significant.
Observation
Morphological analysis
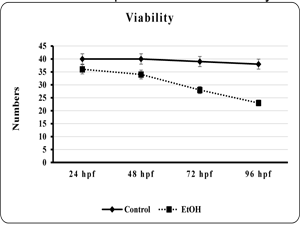
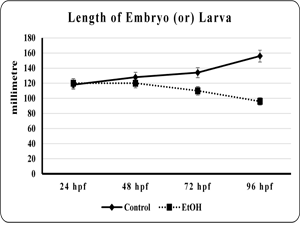
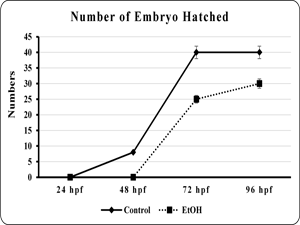
The effect of EtOH on morphology of the developing zebra fish embryo was studied. The viability and total length of the embryo was found to be significantly (p<0.001 and p< 0.001) reduced in EtOH exposed embryo than the control embryo (fig. 1 & 2). Additionally, edematus pericardial sac was observed in EtOH exposed embryo than the control (Plate. 1 & 2). Ethanol exposure significantly (p< 0.001) prolonged the hatching and also lead to embryo malformation when compared to control (fig. 3 & Plate 1).
Effects of EtOH on Heart Morphology
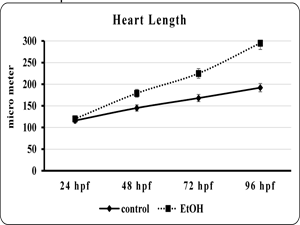
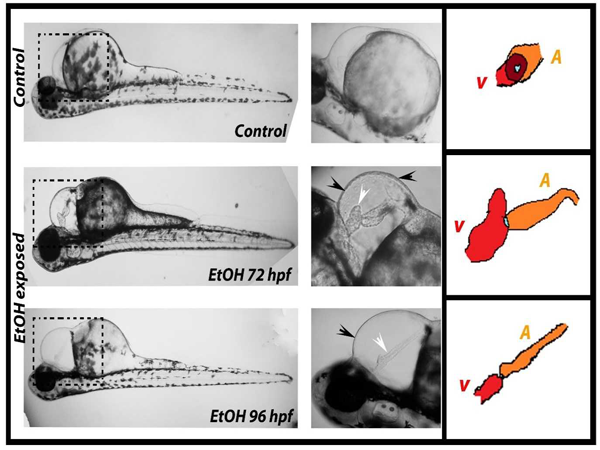
Zebrafish embryos exposed to 3 % EtOH showed pericardial edema and a significant decrease in blood flow by 72 hpf. This change became progressively more pronounced and was quite obvious by 96 hpf. Instead of the looped and S-shaped hearts that was seen in the control fish, hearts from EtOH exposed embryos were found to be elongated and string-like straight tubes (Plate. 2). In control embryos, the normal looping process places the ventricle and atrium side by side, so that the two chambers largely overlap each other in lateral view. In contrast, the EtOH hearts were significantly lengthened (fig. 4).
In the EtOH treated embryos the looping disturbance creates alteration such that the ventricle was positioned anterior to the atrium. Thus, in the EtOH exposed embryos the chambers can be easily distinguished without overlap (Plate. 2). Moreover, in the EtOH exposed animals the atria were thin and elongated and the ventricles appeared smaller and more compact than normal (fig. 4 and Plate. 2).
In order to quantify the effect of EtOH on zebra fish hearts, the distance between the junction of the heart with the inflow tract at the sinus venosus (SV) and the junction with the outflow tract in the region of the bulbus arteriosus (BA) was determined. The resulting numbers provides an index of the change in heart morphology due to the EtOH treatment, and reflect the change in cardiac looping. The EtOH exposure produced significant increases in the BA-SV distance at all the time point when compared to controls (Fig.4 & Plate 2). More specifically, while the control hearts underwent the process of looping and compaction, this process was not observed in the EtOH treated hearts.
Effect of EtOHon Heart Rate
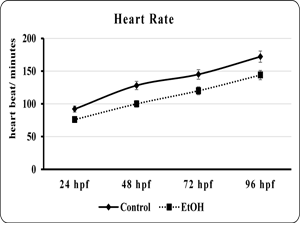
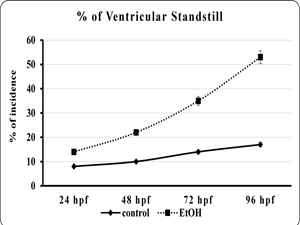
The effects of EtOH exposure on the developing zebra fish heart is not limited morphological alteration. We extend to analyze the heart function by measuring the heart rate and ventricular stand still. The data showed that the EtOH exposure profoundly reduced the heart rate and produce ventricular regurgitation in all the time point when compared to control (fig. 5). The control heart exhibited normal contraction, with the ventricles contracting immediately after the atrium. But the EtOH treated heart showed delays in the contraction of ventricle followed by atrium contraction (fig. 6). This effect is more prominent in 120 hpf were It also found that the 120 hpf were the ventricles had ceased beating almost completely (data not shown).
Peripheral blood flow
The effect of EtOH on peripheral blood flow was analyzed using zebra fish embryo. There is a significant (p<0.001) reduction of blood flow in EtOH exposed embryo (5.5 ± 1.2) when compared to control (23.5 ± 2.10).
Discussion
The effects of EtOH on the developing zebra fish heart are evident by various morphological and functional and developmental defects. These effects include altered looping of heart tube into a string-like or straight tube like appearance, with the ventricle located distinctly anterior to the atrium. Furthermore, the ventricle appears smaller than normal, the atrium is elongated, and both chambers have a narrow width. Interestingly, the EtOH treated heart showed valvular regurgitation of blood and a striking ventricular standstill at all the time point, which clearly demonstrate that teratogenic effect of EtOH on developing heart.
The elongation of the heart by EtOH might be due to EtOH induced failure in morphogenetic movement of cells, which leading to deficiency in attachment point between the heart and the common cardinal vein (CCV) to migrate dorsally, thus mechanically stretching the heart. From approximately 72 to 96 hpf, the heart’s attachment point to the inflow tract migrates dorsally, in a process that appears to contribute to the heart’s looping and compaction within the pericardium (Antkiewicz et al., 2005).
Furthermore, the cardiac morphological defect in heart morphology is more obvious even at 48 hours and it was altered more dramatically in all the experiment time point. Thus, the alteration of heart morphology is present from the beginning of the process of dorsal migration, and therefore cannot be secondary to the disruption of CCV regression. However, the CCV dorsal migration might contribute to the elongated heart morphology at later time points. Similarly, the formation of pericardial edema could move the inflow and outflow attachment points apart, causing the elongated heart morphology. Additionally, EtOH exposure produces the looping impairment which further facilitates the lengthening and alters the inflow and outflow tract (Samoa and Marrs, 2013). Defects in the heart might well be expected to produce a concurrent reduction in circulation. In the present study, we also find that impairment of peripheral blood flow in EtOH exposed embryo when compared to control. This clearly indicated and further emphasized that circulation disturbance plays an additional factor for impairment in structure and function in developing heart (Hove et al., 2003).
The present data clearly shows that EtOH exposure has a striking effect on the ventricles beat (ventricular standstill). Consistent with the present study an earlier report by Bilotta et al., (2004) reveled EtOH exposure to the developing embryo reduces the heart rate. These effects might be due to the increasing pressure (Hove et al., 2003) of the pericardium by pericardial edema, as it could be inhibiting the heartbeat by compressing the heart and preventing filling in a process analogous to cardiac tamponade. We consider this mechanism unlikely because such a mechanism should affect the atrium at least as much as the ventricle, yet the atrial beat is unaffected. However, EtOH exposure might block the impulse propagation at the AV node only if we conclude that the observed phenomenon as an AV conduction block (Antkiewicz et al., 2005). But, the study does not show any progressive arrhythmias, which are commonly associated with AV block. Thus, possible mechanism to explain the ventricular standstill is a failure of the action potential to travel beyond the AV junction. Further, elaborate study is needed to explore how the EtOH alters the AV block and ventricular dysfunction.
Additionally, the valvular regurgitation of blood flow also observed as early as 48hpf in EtOH exposed heart compared to control. It further enhanced in
72 and 96 hpf EtOH exposed heart, in particular, decrease in cardiac output and blood flow becomes more dramatic, the movement of blood cells between the atrium and the ventricle, as well as between the ventricle and the outflow tract can be observed in most embryos. This could be due to an EtOH mediated defect in valve function (Karunamuni et al., 2014) or adaptive response to overcome elevated afterload due to a block in circulation (Hove et al., 2003). Since we could not analyze the vascular flow in 120 hpf due to lack or nil circulation and heartbeat. However, the present study showed retrograde blood flow at 48, 72 and 96 hpf, when blood flow is still substantial and heart contractility seems unaffected. This suggests that the developing valves are not functioning properly, perhaps due to altered valve cushion development, or a loss of contractility at the sites of the nascent valves.
Conclusion
Taken together, the steady decrease in heart size coupled with the severe defects in ventricular function, failure of cardiac looping formation, heart rate, ventricular standstill and retrograde blood flow would be expected to have a substantial impact on the ability of zebra fish to circulate blood. A disruption of heart development in zebrafish embryos exposed to EtOH is particular importance in light of the growing body of evidence demonstrating that the cardiovascular system is also a key target of EtOH toxicity in humans. From the present study it is concluded that EtOH exposure during development results in structural and functional impairment in heart that mimic malformations that occur in patients with fetal alcohol syndrome. Further studies are necessary to identify the molecular mechanism behind how the EtOH affect developing heart.
References
Abel EL, Sokol RJA (1991) revised conservative estimate of the incidence of FAS and its economic impact, Alcohol. Clin. Exp. Res., 15: 514– 524.
Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W (2005) Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol Sci., 84:368-377.
Baumann M, Sander K (1984) Bipartiteaxiation follows incomplete epiboly in zebrafish embryos treated with chemical teratogens. J Exp Zool., 230:363-376.
Bello SM, Heideman W, Peterson RE (2004) 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits regression of the common cardinal vein in developing zebrafish. Toxicol Sci., 78:258-266.
BilottaJ, Saszik S(2001) The zebrafish as a model visual system, Int. J. Dev. Neurosci., 19: 621– 629.
Bilotta J1, Barnett JA, Hancock L, Saszik S (2004) Ethanol exposure alters zebrafish development: a novel model of fetal alcohol syndrome. NeurotoxicolTeratol., 26:737-743.
Carvan MJ 3rd, Loucks E, Weber DN, Williams FE (2004) Ethanol effects on the developing zebrafish: neurobehavior and skeletal morphogenesis. NeurotoxicolTeratol., 26:757-68.
Cole GJ1, Zhang C, Ojiaku P, Bell V, Devkota S, Mukhopadhyay S (2012) Effects of ethanol exposure on nervous system development in zebrafish. Int Rev Cell Mol Biol., 299:255-315.
Daft PA, Johnston MC, Sulik KK (1986) Abnormal heart and great vessel development following acute ethanol exposure in mice. Teratology, 33:93-104.
Duan J1, Yu Y, Li Y, Yu Y, Sun Z (2013) Cardiovascular toxicity evaluation of silica nanoparticles in endothelial cells and zebrafish model. Biomaterials. 34:5853-5862.
Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M (2003). Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature, 421:172–177.
Jones KL, Smith DW, Ulleland CH, Streissguth AP (1973) Pattern of malformation in offspring of chronic alcohol mothers, Lancet, 1:1267–1271.
Mehta V, Peterson RE, Heideman W (2008) 2,3,7,8-Tetrachlorodibenzo-p-dioxin exposure prevents cardiac valve formation in developing zebrafish. Toxicol Sci.,104:303-311.
Sarmah S, Marrs JA (2013) Complex cardiac defects after ethanol exposure during discrete cardiogenic events in zebrafish: prevention with folic acid. Dev Dyn., 242:1184-1201.
Stratton K, Howe C, Battaglia F (Eds.), Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment, National Academy Press, Washington, DC, 1996.
Zar J, (1974) Bio-Statistical Analysis. Prentice Hall Inc. Englewoodcliffs,New Jersey.