International Journal of Anatomical Sciences 2014, 5(1):11-16
Research Paper
Demonstration of Hippocampal Neurons Using Golgi-Cox and Rapid-Golgi Staining Methods – A Report on Their Practical Implications
Ganesh L, Karthik Ganesh M, Anuradha M, Venkata Lakshmi N, Dinesh P, Sakthi Jothi M, Khayinmi WS, Prakash S.
Department of Anatomy, Dr. Arcot Lakshmanasamy Mudaliar Postgraduate Institute of Basic Medical Sciences, University of Madras, Taramani Campus, Chennai 600 113, India.
Key words: Neuro histology, Golgi Cox, Rapid Golgi, Neuron Staining
Abstract: Golgi staining is one of the unique staining procedures to study the morphology of a complete neuron. Random selectivity of only a few neurons to get stained of all the neurons is one of the intriguing facts of this staining method. This empirical randomness questions the veracity of this procedure. To add to this, there are many recipes available to do this procedure. Hence confusions prevail over which method to follow to get a reliable and reproducible Golgi technique of neuronal staining. Present study took the two most popular methods to analyze as follows: A. Rapid-Golgi method (unfixed tissue-immersion fixation) which uses silver nitrate as the base chemical to form the black stain over the neuronal cells. B. Rapid-Golgi method with perfusion fixed tissue and C. Golgi-Cox method using mercuric chloride as the base chemical to form the black staining over neurons. These methods were tried on one of the complex neuronal region of brain i.e. hippocampus. It is observed that Golgi-Cox method seem to give more reliable and appreciable results when compared to that of Rapid-Golgi method.
Hippocampal region of the brain is considered to be one of the hot-seat of today’s neuroscience research if not in the life science research itself. Facts that, comparative simpler organization, limited connections and strongly identifiable individual function make it a widely researched structure for understanding the complex brain in its whole (Andersen et al, 2006). Even though many newer and sophisticated techniques were being developed to visualize this part of the brain, a long practiced, simple but yet powerful technique is Golgi staining (Heinz, 2005). Invented more than a century ago, it lost its importance only to regain in the later part of 20th century after the correlation with memory and the dendritic arborizations in hippocampus were found (Ferrer and Gullotta, 1990). Discovered by Camillo Golgi (Golgi, 1873), widely applied and perfected by Ramon Y Cajal (Cajal, 1909), Golgi staining of neurons brought them their combined Nobel Prize in Physiology or Medicine for the year 1906. This staining helps to visualize the neuronal soma and the processes of the neurons to its whole extent and opened avenues towards understanding the dendritic arborization (Cajal et al., 1999).
The mechanism of this staining procedure falls under silver impregnation technique i.e. by redox reactions (Chan and Lowe, 2002). But the exquisite nature of this procedure lies in the randomness of selection of neurons to get itself stained (Lorente de, 1938). This enables a stained neuron to stand out with all of its process in a clear background. Due to the various factors that could influence this procedure such as pH (Angulo et al., 1994), temperature, duration (Orlowski and Bjarkam, 2009), concentration of the reactants, nature of the tissue, other interferences and contaminants (Spacek, 1992). These variants give great scope for customizing this procedure with application principle, chemicals used, timing and physical factors. Thus many modifications and enhancements were still being tried to complicate and confuse which one to follow (Heinz, 2005). In this study we have tried to compare the A. standard Rapid-Golgi method (unfixed tissue-immersion fixation) which uses silver nitrate as the base chemical to form the black stain over the neuronal cells. B. Rapid-Golgi method with perfusion fixed tissue and C. Golgi-Cox method (Cox, 1891) using mercuric chloride as the base chemical to form the black staining over neurons. Wistar albino rat hippocampus was used for the study.
Materials and Methods
The tissues used in the study were obtained from young male Wistar Albino rats used as control rats for other studies.
Rapid-Golgi method: Under profound sedation rats were decapitated. The skin over the skull was retracted by a midline incision and the brain exposed by cutting the skull open with scissors and forceps. The brain was sliced across the optic chiasma and dorsal to it two slices of 5 mm each was sectioned out with a sharp razor blade.
This method was done as described earlier (Rao and Raju, 2004). The slices were dropped in Rapid-Golgi fixative (Potassium Dichromate-5g, Chloral Hydrate-5g, Gluteraldehyde-8ml, Formaldehyde-6ml, Dimethyl Sulfoxide-10 drops, All mixed in 100ml of Distilled water) kept in an Amber coloured bottle. From second day the tissue was changed to freshly prepared fixative (every time) for the next three days. On the 5th day, the tissue was rinsed with 0.75% aqueous solution of silver nitrate and using a soft brush all deposits over the tissue were wiped.
For the next two days, tissue was kept under 0.75% of silver nitrate solution in a dark place. Tissue was again brushed and then subjected for dehydration with absolute alcohol for 10 minutes and embedded with paraffin wax. Thick sections (>50μm) were taken using rotary microtome (Leica, Germany), sections were collected in a gelatin coated glass slide and cleared with EZ-DeWax TM (BioGenex,USA) or xylene (optional). Sections were mounted with DPX and cover slipped, and examined using light microscope (Nikon Corporation, Japan).
Perfusion fixed Rapid-Golgi method: The animals were given overdose (euthanasia dose) of anesthesia, after cessation of respiration transcardial fixation was performed using 10% formalin (otherwise known as 4% formaldehyde) (Morest and Morest, 1966). All the above procedure (vide supra) for Rapid-Golgi was followed thereafter.
Golgi-Cox method: This method was followed as described by McDonald et al (2005) with modifications in developing the stain. Solutions of 5% potassium dichromate, 5%mercuric chloride and 5% of potassium chromate were prepared and mixed in the ratio of 5:5:4 to 10 parts of distilled water to prepare Golgi-Cox solution (GC solution). Under euthanasia dose, the animals were transcardially perfused with 0.9% saline and the brain was removed. It was cut roughly into three equal parts and the middle part containing hippocampus was immersed in a vial containing GC solution. It was kept in complete darkness and the GC solution was changed during every alternate day for a fortnight. Then the tissue was transferred to a vial containing 30% sucrose and stored at 4°C for a week or so. Then the tissue was sectioned using a vibratome (Leica, Germany) at 200μ thickness and sections were collected in a trough filled with 6% sucrose solution and washed with distilled water. Without exposing much to light, the sections were treated with 22.5% Ammonium Hydroxide solution for 30 minutes and then with 5% Thiosulphate solution for 30 minutes. Then it was rinsed with distilled water for 2 minutes and exposed to light. The sections were collected in glass slides and processed for mounting with DPX and coverslipped for observations.
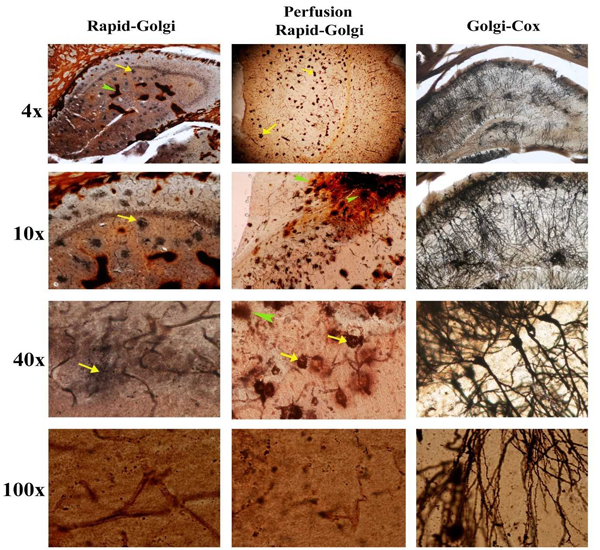
All the above histological preparations were photographed under 4x, 10x, 40x and 100x. The hippocampal regions were analyzed using following parameters viz., background staining of chromating fluid, uniformity of neuronal staining, complete staining of an individual neuron, clarity in staining with dendritic spines and for any artifact.
Observations
In both the Rapid-Golgi methods there were excess chromating fluid staining seen in the hippocampal region. Isolated spots of an orange hue staining were seen in the Rapid-Golgi methods. However, in perfusion fixed Rapid-Golgi method these deposits were less than the standard Rapid-Golgi method. In both these methods, the periphery of the hippocampus that is close to the lateral ventricles showed a thick deposit of the chromating fluid almost masking the visibility of this region. But in the Golgi-Cox method there was a uniform distribution of the background staining and providing a clear visibility throughout the hippocampal region. There were no focal islands of chromating fluid depositions nor specific contrast in the peripheries (Fig. 1).
In Rapid-Golgi methods, focal black deposits were seen but no such over staining were seen in the Golgi-cox method. In the Rapid-Golgi method the soma of the neurons showed intense dark staining (densely aggregated around it). Under high power magnification (100x), the Rapid-Golgi methods showed poor resolution of the dendritic spines but these were clearly appreciable in Golgi-Cox method.
Discussion
In this study we have tried to find the reliable and seamless Golgi neuron staining method by comparing two different methods i.e. Rapid-Golgi and Golgi-cox. It was obvious from our observations that mercuric chloride based Golgi-Cox method was superior than silver nitrate based Rapid-Golgi method. Golgi neuron staining is cherished as one of benchmark methods in staining the neurons and their connections and forms an important tool when comes to correlating with morphological and behavioral neuroscience; however, there are some glitches yet to be resolved. In spite of using these methods for 100 years the exact mechanism of the staining is still not deciphered (Pasternak and Woolsey, 1975). This is considered as an empirical method by some.
A major advantage, as well as the downside in this method are that the randomness of the selectivity for staining particular set of neurons in a given sample, through an unknown mechanism and a factor that cannot be controlled by the investigator; however, completely stains the selected neuron which gives a complete idea on its topography (Angulo et al, 1996). This property to expose the whole neuron is the highlight of this staining method.
There is general consensus that this property or mechanism of staining in the tissue happens from within the neuron and not from outside the neuron (Chan and Lowe, 2002). Overall the neuron which takes the stain is completely stained without any degree of discrepancy or breach, whereas the other neurons which do not take the stain is absolutely unstained and gives a clear background without any interference in viewing the stained neurons.
| Fig.1 Right side long distance of origin of profunda femoris in adult cadavers(More than average distance 3.88cm) | |
|
The available explanation for the principle of Rapid-Golgi staining is that when the tissue is incubated in an aldehyde solution with chromium salts, initially and then exposed to silver nitrate. By a redox reaction, the metallic silver is impregnated in the neuron and gives a black colour (Chan and Lowe, 2002). Whereas in the case of Golgi-cox method the mercuric chloride incubation with chromium salts develops mercurous chloride, which is also known as calomel. On exposure to ammonia, the white coloured calomel changes into black coloured metallic mercury in the neuron (Rosoklija et al., 2014).
The following are the commonly encountered problems in Rapid-Golgi staining
1. Rarely the tissue shows no uptake of color in any of the neuron – reason not known.
2. Relatively dark staining in first few sections and in the peripheries of all the sections.
3. Wherever there is a discontinuity (e.g. para-ventricular regions of the brain) in the tissue, the homogenous distribution of the stain is lost.
4. Mostly there will be patchy aggregation of the black color around the neuronal soma.
5. Even with consistent standardized procedure or time schedule reproducibility of the staining intensity is not guaranteed, which makes it difficult to compare across the experimental groups.
6. The complexity lies in the double incubation of chromating solution followed by silver nitrate solution. With larger tissue slabs which could give room for inconsistent infiltration, resulting in uneven chromation coloring of the tissue. This may be due to factors like texture, make-up and natural partitions of the tissue etc.
Thus the lack of reproducibility in Golgi staining put a question on its reliability (Rosoklija et al., 2003).
While comparing standard Rapid-Golgi method and Perfusion fixed Rapid-Golgi method, the latter one shows relatively better results with an even background, as well as in discrete staining of the neurons and its arborizations. This may be because of the fact that pre infiltrated aldehyde in the fixative increases the penetration of the chromating fluid which also has the aldehydes gluteraldehyde, formaldehyde and chloral hydrate (Angulo et al, 1996). As this accomplishes the initial phase of the process successfully, the chances of hindering the outcome is considerably reduced and so the results are better compared to standard Rapid-Golgi method.
In case of Golgi-Cox method, the ‘calomel’ filled tissues are sectioned by a vibratome, after the first incubation. This increases the penetration of ammonia to the maximum, thus converting all the calomel filled in the neurons to black metallic mercury (Stean, 1974). Subsequent chemical washes stops further reaction and washes the residues leaving behind the metallic mercury in the neuron alone. Thus it gives a uniform staining all around the section and allows us to compare different sections across the experimental groups.
While comparing the Rapid-Golgi and Golgi-Cox methods on the grounds of practical difficulties and applicability, it is obvious that Rapid-Golgi can be done with a simple rotary microtome itself commonly found in any histology lab, whereas Golgi-Cox needs a sophisticated microtome i.e. vibratome for sectioning. However, the chemicals used in Golgi-Cox are readily available when compared to Rapid-Golgi method. The use of chloral hydrate in Rapid-Golgi pose a problem, as it falls under the category of narcotic. Apart from this considerable amount of skill is required in handling the thin sections obtained from vibratome in Golgi-cox method.
Conclusion
In conclusion analyzing the pros and cons of these staining gives insight into practical understanding associated in its usage. One should give due consideration for all the factors to get a successful histological picture using Golgi staining.
References
Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J (Eds.). (2006). Hippocampal Formation : The hippocampus book. Oxford University Press.
Angulo A, Merchan JA, Molina M (1994) Ciolgi-Colonnier method: correlation of the degree of chromium reduction and pH change with quality of staining. J. Histochem. Cytochem., 42: 393-403.
Angulo A., Fernández E, Merchan JA, Molina, M. (1996). A reliable method for Golgi staining of retina and brain slices. J Neurosci Methods., 66:55-59.
Cajal R (1909). Histologie du Systeme Nerveux de l’Homme et des Vertebres, Vols. I and II. Publisher Maloine Paris.
Chan K, Lowe J (2002) Techniques in Neuropathology in Theory and practice of histological techniques JD Bancroft and M.Gamble., 5th edition, Churchill Livingstone, London.
Cox W (1891) Impragnation des centralen Nervensystems mit Quecksilbersalzen. ArchMikr Anat., 37:16–21.
Ferrer I, Gullotta F (1990) Down’s syndrome and Alzheimer’s disease: dendritic spine counts in the hippocampus. Acta Neuropathol., 79:680-685.
Golgi C (1873) Sulla struttura della sostanza grigia della cervello. Gazz Med Ital., Lombardia 6:244–246.
Heinz T (2005) Evolution of the silver and gold stains in neurohistology. Biotech Histochem., 80:211–222
Heinz T (2005) Evolution of the silver and gold stains in neurohistology. Biotech Histochem., 80:211–222.
Lorente de NO R (1938) Synaptic stimulation of neurons as a local process. J. Neurophysiol., 1: 195-206.
McDonald CG1, Dailey VK, Bergstrom HC, Wheeler TL, Eppolito AK, Smith LN, Smith RF (2005) Periadolescent nicotine administration produces enduring changes in dendritic morphology of medium spiny neurons from nucleus accumbens. Neurosci Lett., 9 :163-167.
Morest DK, Morest RR (1966) Perfusion-fixation of the brain with chrome-osmium solutions for the rapid Golgi method. Am J Anat., 118:811-831.
Orlowski DI, Bjarkam CR (2009) Autometallographic enhancement of the Golgi-Cox staining enables high resolution visualization of dendrites and spines. Histochem Cell Biol., 132: 369-374.
Pasternak JF, Woolsey TA (1975) On the “selectivity” of the Golgi-Cox method. J Comp Neurol., 160:307–312.
Ramón y Cajal S, Pasik P, Pasik T (1999) Texture of the nervous system of man and the vertebrates, vol 1. Springer, Wien; New York
Rosoklija G, Mancevski B, Ilievski B, Perera T, Lisanby SH, Coplan JD, et al., (2003) Opti-mization of Golgi methods for impregnation of brain tissue from humans and monkeys. J Neurosci Methods., 131:1–7.
Rosoklija GB, Petrushevski VM, Stankov A, Dika A, Jakovski (2014) Reliable and durable Golgi staining of brain tissue from human autopsies and experimental animals. J Neurosci Methods., 230:20-29.
Shankaranarayana Rao BS, Raju RT (2004) The Golgi techniques for staining neurons. In: Brain and behavior., editors Raju RT, Kutty BM, Sathyaprabha TN, Shankaranarayana Rao BS., Published by NIMHNS, Bangalore, India 108–111.
Spacek J (1992) Dynamics of Golgi impregnation m neurons. Microsc.Res. Techn., 23: 264-274.
Stean JP (1974) Some evidence of the nature of the Golgi-Cox deposit and its biochemical origin. Histochemistry, 40:377–383.
