International Journal of Anatomical Sciences 2011,2(1):1-6
Case Report
Complex Congenital Heart disease with Sacral Agenesis – a Case Report
Srimathi T.
Department of Anatomy, Sri Ramachandra University, Porur, Chennai 600 116, Tamil Nadu, India.
Key Words: congenital heart disease, sacral agenesis
Abstract: Complex congenital heart anomalies are less common. A thirty years old third gravida treated for secondary infertility, gestational diabetes mellitus and hypertension was admitted in Sri Ramachandra Medical College Hospital near term with a breech presentation of the fetus. Labour was induced due to Gestational diabetes and fetal bradycardia. She delivered by vaginal delivery / assisted breech delivery with episiotomy. The child had neonatal respiratory distress, did not cry at birth and was not breast feeding well. The child was treated in the neonatal Intensive care unit for the same. The foetal echocardiogram revealed small left ventricle, dilated right ventricle, single outflow from Right ventricle and probable Truncus arteriosus. Infantogram revealed cardiomegaly and Sacral Agenesis. The case is unique for its rarity and its embryological significance.
Complex congenital heart diseases are less common. A congenital heart defect (CHD) is a defect in the structure of the heart and great vessels that are present at birth either obstruct blood flow in the heart or vessels near it or cause blood to flow through the heart in an abnormal pattern. Congenital heart diseases constitute 1% of all congenital malformations. The cause is genetic in 8% and environmental in 2% of the cases. Mostly it is multifactorial. (Saddler, 2006)
Case Report
Thirty years old female patient was admitted near term .She was married six years ago. She had an induced conception.She was the third gravida with only Live born child in this conception. She had Gestational diabetes mellitus and was on insulin. She also had Pregnancy induced hypertension and was on treatment. Patient was taking antenatal care elsewhere before admission and said to have been taking Folic acid supplements.Her Blood group was Rh negative. Her TORCH PANEL was positive for Herpes simplex virus. There was no positive family history of live births with congenital cardiac anomalies or other birth defects.
Antenatal ultra sonogram of the mother was done and following were the findings:
o At 36 weeks revealed no intrauterine growth retardation, Single live fetus with 36 wks gestation with breech presentation.
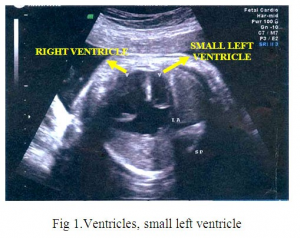
o Fetal bradycardia, small left ventricle(Fig.1)
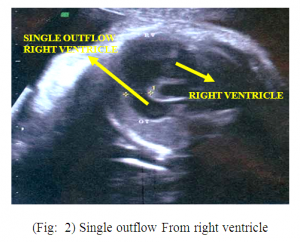
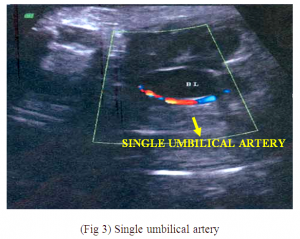
o Single outflow from right ventricle (Fig.2) and Single umbilical artery (Fig.3)
 |
|
|
Patient delivered by assisted breech delivery after induction. Induction was done due to fetal bradycardia and gestational diabetes. A female child was born at term. The cry was weak and the baby did not feed well and had respiratorydistress. Physical examination of the neonate revealed the following findings:
Birth weight was normal with reduced Apgar score (6/10) and normal head circumference and length. There was no cyanosis. The Respiratory rate was increased (76/mt). Oxygen saturation was reduced in arterial blood. Cardiovascular system examination revealed both heart sounds with a pansystolic murmur. Abdomen was soft. Both lower limbs were hypoplastic extended at knees and adducted. As the baby did not cry immediately after birth, assisted ventilation was provided and was treated with intravenous antibiotics after septic workup. The child picked up with normal respiratory effort.
Investigations of the child revealed the following:
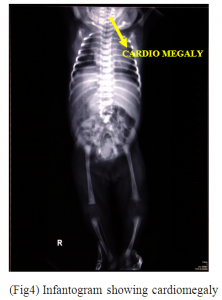
1. Infantogram – Revealed cardiomegaly(Fig.4).
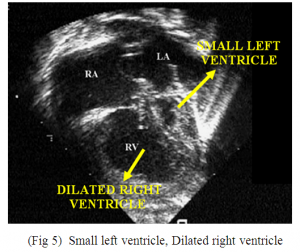
2. Echocardiogram showed Complex congenital heart disease – Hypoplastic mitral ring, Small left ventricle, Dilated right ventricle, Single outflow from Right ventricle (Probable Truncus arteriosus) (Fig.5).
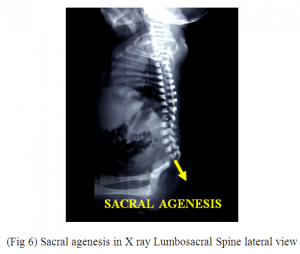
3. X ray LS Spine showed SacralAgenesis (Fig.6)
 |
|
|
Discussion
The clinical features of this case being suggestive of a syndrome called Hypoplastic Left Heart syndrome. This was first described in 1952 by Lev as “hypoplasia of aortic tract” including cases of hypoplasia of aorta, Venticular septal defect, aortic stenosis or atresia, with or without mitral atresia. In 1958 Noonan and Nadas described these lesions as Hypoplastic Left Heart syndrome (Rao,2009). The prevalence of hypoplastic Left Heart syndrome is 0.016-0.27 per 1000 live births. It constitutes 1.4-3.8% of congenital heart disease. The incidence of congenital heart disease as such in India is8 per 1000 live births.
Out of these 180,000 children born each year nearly 60,000 have critical congenital heart disease that requires earlyintervention (Saxena, 2005). The incidence of severe congenital heart disease that will require expert cardiologic care is about 2.5 to 3/1,000 live births. The moderately severe forms of CHD probably account for another 3 per 1,000 live births. (Hoffman, and Kaplan, 2002)
Combination of this syndrome with sacral agenesis is a rare occurrence. Sacral agenesis is rare affecting 1 in 25,000 (Hoffman 1980). While hypoplastic Left Heart syndrome is more common in males but sacral agenesis has equal incidence in both sexes.
The probable causes of the Hypoplastic Left Heart syndrome in this case could be due to abnormal partitioning of the Truncus arteriosus resulting in small aortic outflow tract and a hypoplastic valve annulus. This impedes the blood flow through the aorta from the left ventricle. Since the stimulus for normal development of the aorta is thus interrupted aorta becomes hypoplastic. Normal development of the left ventricle and the mitral valve could have been secondarily affected resulting in hypoplasia of these structures. (Bardo et al., 2001).
The probable causes of sacral agenesis could be due to disorders of primary or secondary neurulation which commonly occurs during the thid to seventh week of pregnancy. (Fellous, et al.), Folic acid deficiency is a probale cause but the patient was taking antenatal care elsewhere before admission and said to have been taking Folic acid supplements. Though she was termed as having gestational diabetes mellitus probable pre gestational undetected diabetes is also a possible cause since the previous blood sugar values were not available. Sacral agenesis may also occur as a part of Curarino’s syndrome .This syndrome is an autosomal dominant disorder with sacral agenesis,mass in thepresacral space, and anorectal mal- formations. This occurs due to mutation of HLXB9 gene most probably microdeletion involving HLXB9.There is a linkage to chromosome 7q36. Homeobox genes (Hox) are required for development of various organs and skeletal develop-ment. Altered patterns of expression or mutations of Hox genes have been shown to produce congenital abnormalities.
It is more likely that the combination of congenital heart disease with sacral agenesis and single umbilical artery, hypoplasia of the limbs in this case is a part of Caudal regression syndrome. The majority of Caudal regression syndrome is sporadic. The role for HLXB9 in caudal regression has not yet been demonstrated, although it is possible that a mutation or DNA sequence variation in the noncoding region of HLXB9 could be present in these individuals, it is more likely that these malformations are due to other factors or defects in other gene(s) (Belloni and Martucciello, 2000). Bernard Duhamel first described the syndrome as a spectrum of congenital malformations, which consist of anomalies of the rectum, the urinary and genital systems, the lumbosacral spine, and the lower limbs. The most severe end of the spectrum is the fusion of the lower limbs and the major organ malformations. This is known as sirenomelia or mermaid syndrome. (Zaw and Stone, 2002)
Caudal regression syndrome is associated with maternal diabetes which is also a possibility in this case. Hyperglyce- mia leads to release of free radicals which can be teratogenic.
Abnormal migration of the neural crest cells is also a probable cause for both the congenital heart disease and the sacral agenesis in this case. Identifying the High Risk mothers for the development of babies with congenital anomalies, proper prenatal screening, possible preventive measures and sometimes intrauterine corrective measures for the birth defect are the only tools to prevent and treat such congenital anomalies.
Identifying High Risk mothers :
Elderly mothers (age 35 and above), diabetic mothers,previous pregnancy with birth defects or genetic disorder, Exposure to teratogens like chemicals, drugs,radiation and TORCH infection,positive family history of genetic disorder, mothers with congenital heart disesases and syphilis are the High Risk mothers for congenital anomalies.(Ganesh Dangal, 2007).
Prenatal Tests:
1. Ultrasonogram
2. Chorion villus sampling
3. Amniocentesis
4. DNA analysis
5. Genetic testing for parents with previous child with birth defects or genetic disorder
6. Routine antenatal screening tests
7. Maternal serum screening for Triple marker and Quad marker (elevation of alfafetoprotein, unconjugated estriol,Human chorionic gonadotrophin and inhibinA) .This test is useful to detect neural tube defects and Down’s syndrome.
8. Preimplantation genetic diagnosis though there are ethical issues .Detects potential defects in an embryo within first few days of conception. A blastomere is removed from embryo and tested for genetic abnormalities in Invitro fertilization.If there are are no such abnormalities then the embryo is implanated.
9. Fetoscopy, Fetal skin sampling, cordocentesis. (Alberman and Berry,1979)
Possible preventive measures:
1. Proper vaccination during pregnacy
2. Folic acid supplements
3. Avoidance of unnecessary medications, alcohol and smoking
4. Consultation with a genetic counselor for the high risk mothers
5. Genetic screening for the high risk mothers
6. Appropriate treatment of maternal diabetes and associated maternal diseases
Intrauterine corrective measures:
Intrauterine corrective surgeries can be done for neural tube defects like spina bifida, congenital heart diseases, sacrococcygeal teratomas and congenital diaphragmatic hernias.,though there are ethical issues. Tworetzky (2004) perfor- med a study in which 3 of 9 fetuses that had a technically successful valvotomy (intra uterine) for aortic stenosis and a continuing pregnancy went on to a 2- ventricle circulation at birth showing Hypoplastic Left Heart Syndrome is preventable in utero
Conclusion:
Once a congenital anomaly is de- tected in the newborn, this should be dis- closed to the family. The nature of the illness and treatment options has to be ex- plained. The family should be helped to accept that this anomaly is predetermined and not the result of birth injury. Counselling should be given. Periodic follow up is mandatory. The treatment options for the hypoplastic left heart syndrome include Norwood procedure, heart transplantation and Supportive expectant Care. (Norwood and Lang 1981) Identifying the High Risk mothers for the development of babies with congenital anomalies, proper prenatal screening, possible preventive measures and some-times intrauterine corrective measures for the birth defect are the only tools to prevent and treat such congenital anomalies.
It is a collective term describing a group of cardiac malformations with various degrees of hypoplasia of the structures of the left side of the heart. Over95% of patients with Hypoplastic Left Heart Syndrome will die if left untreated during the first month of life. Echocardiogram is the diagnostic procedure. As a result of advanced technology, refined surgical techniques (including intrauterine correction), and catheter based interventions, the mortality has reduced for children born with hypop- lasia of the left heart. The syndrome is still considered as one of the most complex congenital cardiac malformations to man- age. Treatment of patients with sacral agenesis is best provided by a team includ- ing an orthopaedic surgeon, urologist, neurosurgeon, pediatrician, physical thera- pist, and onthotist-prosthetist.
Genetic counseling and antenatal diagnosis of such diseases may be the tools for the prevention and management of this disorder. (Connor and Thiagarajan 2007).
The case history and investigations were collected after getting informed consent from the patient (mother).
Acknowledgements
I express my acknowledgements to
Dr. Palaniappan, Dr. Buvana, Dr. Chitra, Department of Obsteterics and Gynaeco-
logy, Sri Ramachandra University, Chennai,
Dr. Sumathylatha, Department of Anatomy, Sri Ramachandra University, Chennai for their guidance.
References
Alberman E, Berry C (1979) Prenatal diagnosis and the specialist in Community medicine. Community medicine, 1: 89-96.
Angelini P (1995) Embrylogy of congenital heart disease. Tex Heart Inst J, 22: 1-12.
Belloni E, Martucciello (2000) Involvement of the HLXB9 Homeobox Gene in Currarino Syndrome. Am J Hum Genet, 66: 1.
Bardo DME, David G. et al., (2001) Hypoplastic Left Heart Syndrome. Radiographics, the journal of continuing medical education, 21:
705-717.
Clark EB (1986) Cardiac embryology: its relevance to congenital heart disease. Am J Dis Child,140: 41-44
Connor JA, Thiagarajan R (2007) Hypoplastic left heart syndrome. Orphanet Journal of Rare Diseases, 2: 23doi:10.1186/1750-1172-2-23.
Dangal G (2007) High-risk Pregnancy. The Internet Journal of Gynecology and Obstetrics, ™ ISSN: 1528-8439 7:
1.Dutta AK (2000) In:Essentials of Human Embryology. 4th Eds. Calcutta: Current Books International. 183Saxena A (2005) Congenital heart disease –A status Report. Ind J Paediatrics, 72:595-598.
Fellous M, Boue J et al., (1982) A five-generation family with sacral agenesis and spina bifida: possible similarities with the mouse T-locus. Am J Med Genet, 12: 465-487.
Hoffman JIE, Kaplan S (2002) The incidence of congenital heart Disease. J Am Coll Cardiol,39: 1890-1900.
Norwood WI, Lang P et al., (1981) Experience with operations for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg, 82: 511-
519.
Pang D, Hoffman HJ (1980) Sacral agenesis with progressive neurological deficit. J Neurosurg,7: 118-126.
Rao SP (2009) Hypoplastic Left Heart Syndrome. eMedicine Specialties > Pediatrics: Cardiac Disease and Critical Care Medicine Cardiology,: 1-64. (http://emedicine. medscape. com / article /890196)
Saddler TW (2006) In : Langman’s Medical Embryology. 10th Edition, Philadelphia, USA: Lippincott Williams & Wilkins. 172.
Tworetzky W, Wilkins-Haug L, et al., (2004) Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation Journal of the American Heart Association, 110: 2125-2131.
Williams DL, Gelijns AC (2000) Surgery for congenital heart disease. J Thorac Cardiovasc Surg, 119: 720-731Zaw W, Stone DG (2002) Caudal Regression Syndrome in twin pregnancy with Type II Diabetes. J Perinatology, 22: 171-174.
