International Journal of Anatomical Sciences 2011, 2(1):11-17
Research Article
Long-Term Hyperglycemic effect on rat Epididymis and Sperm
Suresh S, Shanthi Santhosh Kumari S, Preethi U, Venkatalakshmi N, Karthik Ganesh M,Ganesh L, Prithiviraj E, Prakash S.
Department of Anatomy, Dr. Arcot Lakshmanasamy Mudaliar Postgraduate Institute of Basic Medical Sciences, University of Madras, Taramani Campus, Chennai 600 113, INDIA.
Key Words: hyperglycemia, epididymis, sperm
Abstract: The epididymis, an important organ of male reproduction, imparts sperm maturation acquiring motility and fertilizing potential and removal of defective sperm and providing good microenvironment. In addition the epididymis plays a significant role in the transport, concentration, protection, and storage of spermatozoa. The objective of the present study was to analyse the patho-physiological changes in epididymis, sperm morphology, count, maturation and transport under long-term hyperglycemic condition using Wistar Albino rats as diabetic (hyperglycemic) model. Twenty male Wistar albino rats (Rattus norvegicus) were used for this study (body weight 225– 250 g) The rats were randomly selected for the following groups i.e. group I – control (received 0.1M Citrate buffer) and group II – Diabetes (single injection of streptozotocin 60 mg/kg b.w. in 0.1M citrate buffer). At the end of 120 days animals were sacrificed by over dose of anesthesia (i.p), and followed by transcardial perfusion using 4 % paraformaldehyde in 0.1 M phosphate buffered saline. Epididymides were dissected out and after gross measurements were taken, processed for paraffin technique. Sections were stained using hematoxylin (Harris’s) and eosin and observed under light microscope and histomorphometry and stereological analyses were done. Spermatozoa were collected from caudal portion of epididymis to estimate sperm viability and motility, morphology and morphometry, cytoplasmic droplets and epididymal transit time. The present data revealed major damage in structure of epididymis and altered sperm quality and quantity under the influence of long-term diabetes. The alteration in epididymis might have influenced the sperm damage and poor sperm parameters leading to infertility. With the age limit to acquire diabetes is coming down rapidly and thus increasing the risk of infertility in younger generation. Consequently, more number of basic and clinical researches should be focused on the prevention and cure of diabetes.
Infertility in males is a topping list of male related sexual problems nowadays. It affects as many as one in six couples, the possible causes comes under the following
Idiopathic, Varicocele, ageing, hormonal imbalance, Substance Abuse (alcohol, nicotine), Testicular factors, sedentary lifestyles, environmental factors, thyroid disease, hypertension, heart disease and diabetes mellitus (DM). DM may affect male reproductive function at multiple levels including spermatogenesis as a result of its endocrine impairment (Baccetti et al., 2002; Ballester et al., 2004). Animal studies using rodent models of streptozotocin-induced diabetes mellitus have demonstrated a reduction in sperm count and quality (Ballester et al., 2004; Amaral et al., 2006; Scarano et al., 2006). Human diabetic study showed reduction in all semen parameters (semen volume, sperm count, motility and morphology) (Garcia-Diez et al., 1991).
The epididymis, an important organ of male reproduction, imparts sperm maturation acquiring motility and fertilizing potential and removal of defective sperm and providing good microenvironment (Turner, 2007). In addition to sperm maturation, the epididymis plays a significant role in the transport, concentration, protection and storage of spermatozoa and all these process are androgen dependent (Meistrich et al., 1975).
The objective of the present study was to analyse the patho-physiological changes in epididymis and the sperm morphology, count, maturation and transport under long-term hyperglycemic condition using Wistar Albino rats as diabetic (hyperglycemic) model.
Materials and Methods
Animal Used
Twenty male Wistar albino rats (Rattus norvegicus) were used for this study. Male rats of body weight (b.w.) 225– 250 g were selected. They were housed individually in separate standard cages and maintained under standard laboratory conditions (temperature 24–28°C, relative humidity 60–70%, and 12-hour light–dark cycle) with free access to solid pellet diet and water ad libitum throughout the study. The study was approved by Institutional Ethical Committee. Quarantine procedures and animal maintenance was according to the recommendations of Canadian Council Guide to the Care and Use of Experimental Animals (1993) and Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) guidelines for laboratory animal facility (2003). Experimentation on these animals carried out after subjecting to quarantine period of not less than 15 days each.
Experimental Design and Induction of Diabetes
In this present study, animals were grouped as, group I – control (received 0.1M Citrate buffer) and group II – Diabetes (single injection of streptozotocin (Sigma- Aldrich, USA) 60 mg/kg b.w. in 0.1M citrate buffer). At the 4th day after injection the animal blood glucose level were estimated. Animals which showed above 250 mg/dl were considered as diabetic (Suresh and Prakash, 2010).
Tissue Harvesting
At the end of 120 days after recording final body weights, animals were sacrificed by over dose of anesthesia (i.p), and followed by transcardial perfusion using 4 % paraformaldehyde in 0.1 M phosphate buffered saline. Epididymides were dissected out and gross measurements were taken. The tissues were fixed for histological studies in freshly prepared Bouin’s fluid and processed for paraffin technique. Sections were stained using hematoxylin (Harris’s) and eosin and observed under light microscope (Nikon. Japan)
Sperm Analysis
Sperm count:
Sperm count was done according to the procedure described previously (Suresh et al., 2010). Briefly spermatozoa were collect from caudal portion of epididymis, by mincing caudal epididymis with anatomical scissors in 5 ml of pre warmed (35º C) physiological saline, placed in a rocker for 10 min. and incubated at room temperature for 2 min. Supernatant fluid was diluted 1:100 with solution containing 5 g Sodium bicarbonate, 1 ml formalin (35 %) and 25 mg Eosin/ 100 ml H2O. Total sperm count was determined using haemocytometer. Approximately 10μl of diluted sperm suspension was transferred to each counting chamber and was allowed to stand for 5 min and then counted under a light microscope at 400 X magnification.
Sperm Viability and Motility:
About 20μl of sperm suspension was mixed with an equal volume of 0.05% eosin- Y and nigrosin. After 2 min incubation at room temperature, slides were viewed by bright-field microscope with 400 X magnification (Nikon, Japan). Dead sperms appeared pink and live sperms were not stained (Suresh et al., 2010). Percentage of motile sperm was assessed using graded semi-quantitative scale of 0 to 5 and the spermatozoa were evaluated for the rate of forward movement and graded accordingly, i.e., 0 = No movement, 1= Sluggish or tail movement alone, 2 = Intermittent sluggish movement, 3 – 4 = Fair & Good movement and 5 = Maximum movement in forward direction.
Morphology and Morphometry:
The fixed sperm were smeared on a glass slide and stained with phosphate buffered saline solution of Giemsa (Merck, Germany) (Hafez, 1977). Morphological alterations in sperm and morphometrical data acquired by measuring length of the head and flagellum were evaluated. A total of one hundred spermatozoa were analyzed per animal using ocular micrometer scale fitted to a light microscope under 40X magnification.
Cytoplasmic Droplet Containing Sperm
The presence of cytoplasmic droplet containing sperm was determined using the method of Syntin & Robaire (2001). Spermatozoa were assessed for the presence or absence of cytoplasmic droplet, for which minimum of 100-spermatozoa/ animal were evaluated.
Estimation of Epididymal Transit Time
The epididymal transit time were calculated according to the methods of Scarano et al., (2006). Briefly the epididymal sperm count were calculated in individual region (Caput, corpus and caudal). Then transit time of caput/corpus and caudal epididymis were calculated by dividing the number of sperm within each of these three regions by the daily sperm production.
Histomorphometry and stereological analyses
The conventional stereological principles and accepted morphometric procedures as outlined by Elias and Hyde, (1980) were used to obtain quantitative information, details of the procedure have been described previously (Prakash et al., 2008). Systematic unbiased random sampling (SURS) protocol was adopted. All the data’s are expressed in relative value.
Statistical analysis
The significant difference between the mean value of control and experimental groups was determined by student‘t’ test. P value <0.05 was considered as statistically significant (Zar, 1974). All the analysis was done by using Microsoft Excel 2003 and SPSS statistical package version 7.
Observations
Sperm analysis
Significant reduction in percentage of viable and increase of the dead sperm were observed in the long term hyperglycemic rats when compared with normoglycemic rats. There was significant decline in motility in hyperglycemic when compared with normoglycemic rats.
The sperm concentrations and motility were markedly decreased in the long term hyperglycemic rats. No significant changes were observed in the morphometry study in all the experimental groups. Morphological analysis shows wide degree of abnormality in long term diabetic rat sperm such as defects in the head (microcephalic, bicephalous, amorphous, and acephalic), neck and tail when compared to the group I.
Cytoplasmic droplet containing sperm
Cytoplasmic droplet study showed significant increase of the sperm containing cytoplasmic droplets in long term hyperglycemic rats (45 ± 1.12) when compared to normoglycemic rats (3 ± 0.39). These cytoplasmic droplets were located at various levels in the sperm; tail remnant was more in number. Observations are summarized in table 2.
Epididymal transit time
Significant reduction in the epididymal transit time was observed in the long term diabetic rat when compared with normal rats. Data’s were shown in table 1.
Table 1 Sperm analysis data and epididymal transit time of control and long term diabetic animals. Each value indicates the mean ± SD of (n – 6) animals. a – control, *** – p<0.001.
| PARAMETER | CONTROL | DIABETES | |
| Sperm viability (%) | |||
| Live | 98 ± 1.23 | 60 ± 2.12 a*** | |
| Dead | 2 ± 0.89 | 40 ± 1.65 a*** | |
| Motility (%) | 98 ± 2.15 | 50 ± 1.86 a*** | |
| Count (106) | 371 ± 12.02 | 128 ± 3.25 a*** | |
| Morphology (%) | |||
| Normal | 96 ± 2.12 | 67 ± 3.20 a*** | |
| Abnormal | 4 ± 0.35 | 33 ± 1.32 a*** | |
| Epididymal sperm count (X106/organ) | |||
| Caput/ Corpus | 123.67 ± 4.68 | 45.23 ± 2.17 a*** | |
| Caudal | 247.33 ± 2.63 | 82.77 ± 1.86 a*** | |
| Epididymal sperm transit time (days) | |||
| Caput/ Corpus | 6.110 ± 0.25 | 2.47 ± 0.86 a*** | |
| Caudal | 12.22 ± 1.01 | 4.52 ± 1.06 a*** | |
Epididymal Morphological Changes
There were morphological alteration such as decrease in the length, breadth, height, weight and volume in all the three regions of epididymis i.e. caput, corpus and cauda of long term hyperglycemic rats when compared to the normoglycemic rats. Data’s were shown in table 2)
Table 2 Morphology of epididymis in control and long term diabetic animals. Each value indicates the mean ± SD of (n –6) animals. a – control, * – p<0.05, ** -p<0.01 and *** – p<0.001
| PARAMETER | Control | Diabetes |
| Length (cm) | 5.33 ± 0.24 | 4.1 ± 0.15 a ** |
| Breadth (cm) | ||
| Caput | 0.63 ± 0.12 | 0.25 ± 0.11 a** |
| Corpus | 0.3 ± 0.10 | 0.13 ± 0.09 a* |
| Caudal | 0.85 ± 0.08 | 0.39 ± 0.11 a** |
| Width (cm) | ||
| Caput | 0.53 ± 0.12 | 0.15 ± 0.09 a*** |
| Corpus | 0.15 ± 0.03 | 0.1 ± 1.59 a* |
| Caudal | 0.54 ± 0.06 | 0.24 ± 0.08 a** |
| Volume (ml) | 0.48 ± 0.11 | 0.15 ± 0.09 a** |
| Weight (gm) | 0.643 ± 0.06 | 0.31 ± 0.03 a*** |
Histological observation
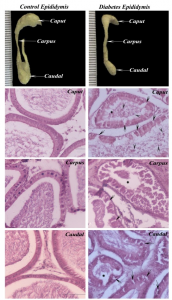
Epididymal histology shows the thickening of basement membrane, vacuolation of the epithelial cells and absence of mature spermatozoa and presence of immature germ cell in the lumen of tubules in the long term diabetic animals when compared to the control (Fig. 1).
Histomorphometric Changes
There was significant change in volume of connective tissue, tubule and epithelia. The number of tubule was found to be increased and the diameter found to be decreased in diabetic group (Figure 2 to 7).
Discussion
The present data’s revealed that major changes in structure and functions of epididymis plays a significant role in altering sperm quality and quantity under the influence of long-term diabetes. This includes reduction in gross weight and volume of the epididymis concomitant with decrease in the volume of spermatozoa in the tubular lumen may be partly accounted for the reduction in epididymal weight and volume in the diabetic rats. This may be due to the impaired spermatogenesis in diabetic condition (result not shown).
Fig. 1 Morphology and histology of the epididymis in control and long term diabetic rats. In diabetic animal (arrow) showing vacuole formation in the epithelium Arrow head indicates the immature germ cells in the lumen and the star indicates the occluded lumen.
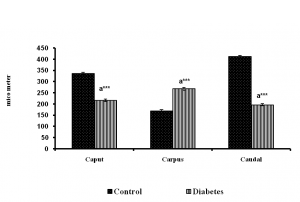
Fig. 2 Diameter of the epididymal tubules of control and long term diabetes animals.
Fig. 3 Height of the epididymal epithelium of control and long term diabetes animals. Each bar indicates mean ± SEM of (n-6) animals. a – control, * – p< 0.05, ** –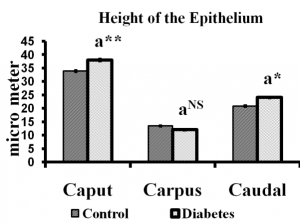
Fig. 4 Volume of connective tissues of control and long term diabetes animals. Each bar indicates mean ± SEM of (n-6) animals. a
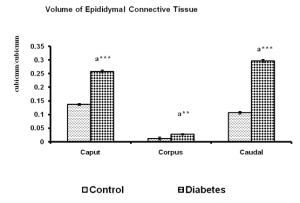 |
Fig. 5 Volume of epididymal epithelium of control and long term diabetes animals. Each bar indicates mean ± SEM of (n-6) animals. a – control, *** – p< 0.001.
|
|
Fig. 6: Volume of epididymal tubules of control and long term diabetes animals. Each bar indicates mean ± SEM of (n-6)animals. a – control, ** – p<0.01 and *** – p< 0.001. shows that epididymal dysfunction and disturbed homeostasis. The pathology could have been enhanced by the impaired spermatogenesis and immature spermiation which produce immature or sperm with cytoplasmic droplets (Suersh and Prakash,2010).
The changes in epididymis had severely affected sperm parameters when compared to control. The sperm count and motility were decreased with increased number of abnormal sperm in the hyperglycemic epididymis. The sperm with Fig 7 Number of the epididymal tubules of control and long term diabetes animals. Each bar indicates mean ± SEM of (n-6) animals. a – control, * – p<0.05 and *** – p< 0.001. cytoplasmic droplet was also more in hyperglycemic rat indicating patho- physiological state of the testis and epididymis. The presence of sperm with cytoplasmic droplet can increase free radical production and oxidative stress in the epididymis, a condition seen in our previous study on aged rat epididymis (Suresh et al.,2010).
The histological observation of diabetic epididymis showed vacuolation, epithelial fibrosis and lumen filled with immature and degenerative sperm cells, demonstrating the deleterious effect of long-
In general epididymis depends on androgens to maintain their structure and function (Amman et al., 1993). The morphological alteration seen in diabetic epididymis may be predominantly due to the androgen deficiency. The serum testoste- rone and luteinizing hormone were reduced in diabetic condition as observed in our previous study (Suresh and Prakash, 2010). Usually in normal rats deprived of testosterone, there will be increase in the rate of epididymal sperm transit time (Meistrich et al., 1975). However, in this study the diabetic rats deprived of testosterone showed significant decrease in epididymal transit time for sperm. There was increased amount of immature and abnormal sperm in long term diabetic rats when compared with control rats. Present observations clearly term hyperglycemia. Histomorphometeric observations showed more number of tubules per unit area. This might be due to the shrinkage of tubular diameter. Similarly this shrinkage or concentration of more tubules might have caused increase in epithelial height and volume. There was increased amount of connective tissue proportion.
These structural changes suggested that the epididymal physiology could have severely altered and consequently, resulted in sperm damage which may lead to infertility. With the age limit to acquire this disease is coming down rapidly and thus increasing the risk of infertility in younger generation. Given the finite nature of the human existence, an individual man’s most precious and lasting gift to the world is the legacy created by his offspring. The most unfortunate thing is that India will be the diabetes capital of the world in 2030 according to a World Health Organization (WHO) report. Hence, more basic and clinical research is required for the prevention and cure of diabetes in India.
References
Ali ST, Shaikh RN, Siddiqi NA (1993) Semen analysis in insulin-dependent/non-insulin- dependent diabetic men with/without neuropathy. Arch Androl, 30: 47–54.
Amaral S, Moreno AJ, Santos MS, Seica R, Ramalho-Santas J (2006) Effects of hyperglycemia on sperm and testicular cells of Goto-Kakizaki and streptozotocin-treated rat models for diabetes. Theriogenology, 66: 2056–2067.
Amman RP, Hammerstedt RH, Veeramachaneni DNR (1993) The epididymis and sperm maturation: a perspective. Reprod Fert Dev, 5:361–381.
Baccetti B, Marca Al, Piomboni P, Capitani S, Bruni E, Petraglia F, De Leo V (2002) Insulin- dependent diabetes in men is associated with hypothalamo-pituitary derangement and with impairment in semen quality. Hum Reprod, 17:2673–2677.
Ballester J, Muñoz MC, Domínguez J, Rigau T, Guinovart JJ, Rodríguez-Gil JE (2004) Insulin- dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl, 25:706–719.
Elias H, Hyde H (1980) An elementary introduction to stereology. Am J Anat, 159: 411–446.
Cameron DF, Rountree J, Schultz RE, Repetta DF, Murray T (1990) Sustained hyperglycemia results in testicular dysfunction and reduced fertility potential in BBWOR diabetic rats. Am J Physiol, 259: E881–E889.
Committee for the Purpose of Control and Supervision on Experiments on Animals CPCSEA guidelines for laboratory animal facility (2003) Committee for the Purpose of Control and Supervision on Experiments on Animals. Ind J Pharmacol, 35: 257–274.
Olfert ED, Cross BM, McWilliam AA (1993) Guide to the care and use of experimental animals. Volume 1. 2nd ed. Canadian Council for Animal Care.
Frenkel GP, Homonnai ZT, Drasnin N, Sofer A, Kaplan R, Kraicer PF (1978) Fertility of the
streptozotocin-diabetic male rat. Andrologia, 10:127–136.
García-Díez LC, Corrales Hernandez JJ, Hernandez- Diaz J, Pedraz MJ, Miralles JM (1991) Semen characteristics and diabetes mellitus: significance of insulin in male infertility. Arch Androl, 26:119–128.
Hafez ESS (1977) Techniques of human andrology.
Published by Elsevier/North- Holland Biomedical press. 471. Amsterdam, Netherland.
Handelsman DJ, Conway AJ, Boylan LM, Yue DK, Turtle JR (1985) Testicular function and glycemic control in diabetic men. A controlled study. Andrologia, 17: 488–496.
Meistrich ME, Hughes TH, Bruce WR (1975) Alteration of epididymal sperm transport and maturation in mice by oestrogen and testosterone. Nature, 258: 145-147.
Mori H, Christensen AK (1980) Morphometric analysis of Leydig cells in the normal rat testis. J Cell Biology, 84: 340-354.
Phil-Ok Koh (2007) Streptozotocin-Induced Diabetes Increases Apoptotic Cell Death in Rat Epididymis. Lab Anim Res, 23: 381-384.
Prakash S, Prithiviraj E, Suresh S (2008) Development of seminiferous tubules in Prenatal, Postnatal and adult Testis of Bonnet Monkey (Macaca radiata). Anat Hist Emb, 37: 19 – 23.
Syntin P , Robaire B (2001) Sperm structural and motility changes during aging in the brown Norway rat. J Androl, 22 : 235–244.
Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Kempinas WG (2006) Sexual behaviour, sperm quantity and quality after short-term streptozotocin-induced hyperglycaemia in rats. Int J Androl, 29: 482–488.
Sexton WJ, Jarow JP (1997) Effect of diabetes mellitus upon male reproductive function. Urology, 49: 508–513.
Suresh S, Prithiviraj E, Prakash S (2010) Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. Int J Androl, 33: 22-32.
Turner TT (2008) De Graaf’s Thread: The Human Epididymis. J Androl, 29: 237-250.
Vignon F, Le Faou A, Montagnon D, Pradignac A, Cranz C, Winiszewsky P, Pinget M (1991) Comparative study of semen in diabetic and healthy men. Diabete Metab, 17: 350–354.
Zar JH (1974) Biostatistical analysis. Engle wood cliffs NJ; Prentice Hall Inc.