International Journal of Anatomical Sciences 2013, 4(2):03-10
Research Paper
Ageing Induced Changes in Ventricular Myocardium: A Histological and Histomophometrical Study
Sekar Suresh, Anandan Balakrishnan, Raziya Banu, Seppan Prakash.
Department of Anatomy, Sathyabama University, Chennai – 600 119, India.
Department of Genetics & Molecular Biology, University of Madras, Taramani Campus, Chennai – 600 113, India
Department of Anatomy, Karpagavinayaga Institute of Medical Sciences & Research, Kanchipuram – 603 308, India.
Department of Anatomy, University of Madras, Taramani Campus, Chennai – 600 113, India.
Key words: Ageing effects – myocardial changes
Abstract: The objective of the present study is to evaluate the ageing mediated alteration in the cardiac tissue by histological and histomorphometrical prospective. The Wistar albino rats were divided in to two groups. Group I – Young (3-5 months) and Group II – Aged (28 months). Animals were sacrificed with transcardial perfusion with 4 % paraformoldehyde in PBS (pH 7.2) and heart was dissected out and post fixed in same fixative. The tissue was subjected to various analyses like morphological, histopathological, histomorphometrical, fibrotic changes (Trichrome staining) and apoptotic cell death (DAPI staining). The result showed significant increase in the size (p<0.01), weight (p<0.01) and volume (p<0.01), left ventricular wall thickness (p>0.001) and diameter of ventricular cavity (p<0.001) of the aged animal when compared to the control. The histological observation of aged rat heart showed marked alteration like hypertrophy of the cardiomyocytes, lymphocyte infiltration and the pyknotic nucleus and increased collagen deposition (tichrome staining). Histomorphometrical study showed significant increase in diameter (p<0.001) and volume of muscle fiber (p<0.001) with reduction of numerical density (p<0.001) in aged heart when compared to control. From the study it is hypothesized that cardiac hypertrophy, fibrosis and apoptosis in ventricular myocardium might be due to overload induced maladaptive response. Whether this aging-associated event progresses with senescence and becomes the most significant factor for the development of heart failure in the elderly requires further investigation.
Aging is the natural phenomenon, which is the process of growing old and is usually defined as the gradual biological impairment of normal function which has direct impact on the functional ability of organs and on the biological systems. These irreversible series of changes inevitably end in death. The numbers of aged people in North America are going to be double in next 25 years. In India it will increase from 76 million to 131 million in the period between 2001 – 2021. As the number of people over age 65 in North America is expected to double over the next 25 years. India it will increase from 76 million in 2001 to 137 million by 2021 and having second largest aged population in the world.
Progressive fibrosis is a hallmark of aging in various organs such as kidney (Gagliano et al., 2000), liver (Gagliano et al., 2007), pancreas (Glaser and Stienecker, 2000) and lung (Calabresi et al., 2007). In the cardiovascular system a progressive age-related deposition of collagen in the vascular wall and in the cardiac interstitial and perivascular space leads to reduction of myocardial and arterial compliance (Lakatta and Levy, 2003). Cardiac aging is associated with diastolic dysfunction and heart failure with preserved systolic function (Lakatta and Levy, 2003; Kitzman et al., 2001). Some have demonstrated overt systolic dysfunction with age (Biesiadecki et al., 2010), but others have not (Barouch et al., 2003; Ma et al., 2010). Still other groups have shown that aging is associated with normal baseline cardiac function, but reduced cardiac response to inotropic stimuli (Lakatta and Sollott, 2002).
Furthermore, ageing mediated cardiomyocyte apoptosis and autophagy to the cardiomyopathy of aging have not been fully described. To describe and quantify these pathologies is the first step toward developing disease-specific approaches to the treatment of age-related cardiomyo-pathy. Though number of studies has been reported, the exact mechanism of ageing dependent collagen accumulation and their relation to apoptosis of cardiac myocytes is still unclear. Thus the present study was to evaluate the association of ageing induced ventricular fibrosis and apoptosis and their role in cardiac hypertrophy.
Materials and Methods
40 thigh specimens from adult human cadavers and 10 thigh specimens from dead born fetuses were made use of. Conventional dissection method was used for the study.
Observations
Animals
Animals were maintained as per the national guidelines and protocols, approved by our institutional ethical committee (IAEC No. 01 ⁄ 031 ⁄ 05). Healthy male Wistar albino rats (Rattus norvegicus) are divided into four groups: group I – control (young weighing 175–200 g 3–6 month); group II – aged (weighing 425 – 450 g 26 – 28 month old rats). The animals was maintained in 12 h light–dark cycle under controlled conditions and in room temperature (23 ± 2 ⁰C), humidity (50 ± 5%). The animals were fed with standard rat pellet diet and drinking water ad libitum. The animals were sacrificed by trancardiac perfusion.
Tissue harvesting Morphology and histological study
At the end of experimental period animals were sacrificed by over dose of anesthesia (i.p), and followed by transcardial perfusion using 4 % paraformaldehyde in 0.1 M phosphate buffered saline. Hearts were dissected out and gross measurements (like size, weight, volume, thickness of ventricular wall and diameter of ventricular cavity) were measured. The tissues were postfixed in 4 % paraformaldehyde till the further analysis. A small piece of ventricular tissue was cut and it was processed for paraffin technique. Sections were stained using hematoxylin (Harris’s) and eosin and observed under light microscope (Nikon. Japan).
Histomorphometry and stereological analyses
The conventional stereological principles and accepted morphometric procedures as outlined by Elias and Hyde, (1980) were used to obtain quantitative information, details of the procedure have been described previously (Prakash et al., 2008). Systematic unbiased random sampling (SURS) protocol was adopted. All the data’s are expressed in relative value.
Masson Trichrome staining
The sections were subjected to Trichrome staining by the methods described by suresh and Prakash, (2011). The sections were deparaffinized in xylene and were dehydrated in graded alcohol and brought to distilled water. The nuclei were stained with Celestin blue hematoxylin methods and differentiated with 1% acid alcohol then washed well in tap water. After that the slides stained with acid fuchsin solution (acid fuchsin, glacial acetic acid and distilled H2O) for 5 min and rinsed in distilled water. Treat the sections with phosphomolybdic acid solution for 5 min, drain the excess solution from the sections and then stain with methyl blue (Methyl blue, glacial acetic acid, distilled H2O) for 3 min, treat the section with 1 % acetic acid for 2 min after washing with distilled water. Sections were dehydrated through alcohol, cleared in xylene and mounted with DPX.
Apoptotic study by DAPI staining
The apoptotic changes were studied by chromatin staining with DAPI. The paraffin sections were deparaffinized in xylene, dehydrated in graded alcohol and brought to distilled water and finally exposed to DAPI (2 µg/ ml) for 10 min at room temperature. The excess stain was removed by washing with PBS twice 5 min each and examined under ultraviolet illumination with a fluorescence microscope (Olympus Optical, Tokyo, Japan).
Statistical analysis
The significant difference between the mean value of control and experimental groups were analyzed according to the method of Zar (1974). All the data were subjected to Student’t’ test and data showing p value <0.05 was considered as statistically significant.
Observations
Morphology and histological study
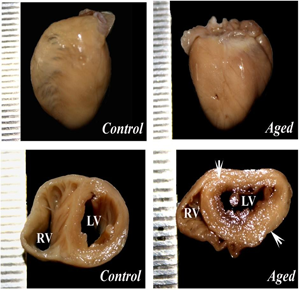
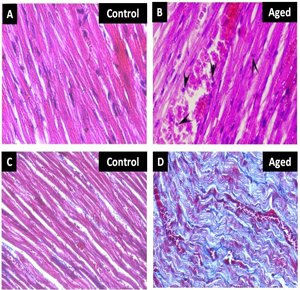
Ageing mediated alteration in the morphology of the heart was studied. The data indicates that there was a significant increase in the size, volume (p<0.01) and weight (p<0.01) of the aged heart when compared to control. However the ventricular wall thickness was also found to be significantly (p<0.001) increased with concomitant reduction of ventricular cavity diameter in aged animal compared to control (Fig. 1). The histological of aged heart showed marked alteration like hypertrophy of the cardiomyocytes, neutophile infiltration and pyknotic nucleus when compared to young control (Fig. 2A & 2B).
Histomorphometrical study
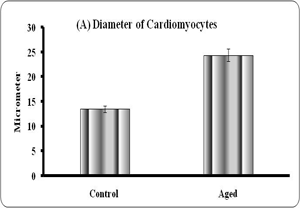
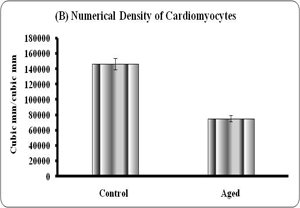
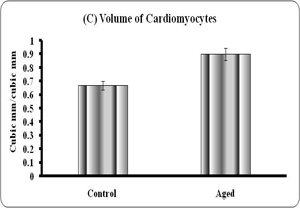
Quantitative analyses of histological changes were done by the means of hisomorphometrical analysis. The result showed that the aged heart showed significant increase in diameter (p<0.01), volume (0.001) and significant reduction in numerical density (p<0.001) of the fibers when compared to control (Graph – A to C).
Graphs: Histomorphometry of cardiomyocyte in young and aged heart tissue. Each bar indicates mean ± SEM of n=6 animals. ‘a’ – control, ** – P< 0.01 and *** - p<0.001.
Massion’s trichrome
The fibrotic changes in the heart were evaluated by special staining (massion trichrome). The data indicates that the young control heart showing negative for the trichrome staining but the aged animal showed more positive sites for trichrome. The positive sites are represented as presence of collagen fiber which is stained by blue, nucleus are stained by hematoxilin and the cytoplasm are stained by the brown (Fig. 2C & 2D).
Apoptotic study by DAPI staining
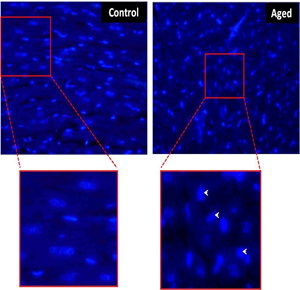
Ageing mediated cardiac myocyte apoptosis was studied by 4,6- diamidino-2-phenyl indoledihydrochloride (DAPI) staining. Both qualitative and quantitative study indicates that the aged heart showed significant increase in nucleus with apoptotic morphology when compared to control (Fig. 3).
Discussion
Ageing mediated structural and functional impairment of body systems is one of the leading health problems in the elderly population which finally leading to mortality. In the present study increased overall heart size was observed in aged animal particularly the left ventricle is showing marked alteration compared to right ventricle.
Figure 1 (A & B): showing the morphology of the heart in young and aged animal. The panel of C & D showing the cross section of the ventricles of control and aged heart. The arrow indicates that the increased wall thickness of in left ventricle of aged heart. RV – Right ventricle and LV – Left ventricle
Figure 2 (A & B): showing the histology of heart in young and aged heart tissue stained with H & E. The 2 (C & D) showing the heart tissue section stained with trichrome staining. The blue colour indicates the collagen fibres and the red indicates the muscle fibre
Figure Nuclear morphology of the rat myocardium in control and aged heart tissue stained with DAPI. The aged myocardium showing the apoptotic nuclear morphology (arrow) compared to control
These changes might be due to the maladaptive process induced by biomechanical stress, be it extrinsic, such as in arterial hypertension or valvular heart disease, or intrinsic, as in familial hypertrophic cardiomyopathy (Frey and Olson, 2003). Furthermore, the increasing blood volume with body mass can be expected to result in an increased volume load on the heart, a condition in which an identical additional work demand is applied to both ventricles. However, the left ventricle is subjected to a greater pressure load because systemic arterial pressure is significantly higher than pulmonary pressure (Anversa et al., 1986; Isoyama and Nitta-Komatsubara, 2002). The combination of these effects on the left ventricle associated with a deficit in the coronary microcirculation may exceed its compensatory capacity, leading to myocyte necrosis.
Histological study increased the hypertrophy of the cardiac myocytes and neutrophil infiltration and the pignotic nucleus was observed in the aged animals when compared to young. These histological changes might be the normal ageing changes. However the lymphocytes infiltration clearly indicates that ageing mediated inflammatory reaction which is mainly due to increased serum cholesterol levels thereby it stimulate hypertension (Omoigui, 2007; Cieslik et al., 2011) which ultimately leading to increase work load to the cardiac tissue. As a result of overload the ventricular tissue increase their size as adaptive mechanism but it is maladaptive leads to hypertrophy of the muscle fiber. Furthermore, the ageing heart also showing the pyknotic nucleus which might be due to the increased inflammation reaction and the inflammation mediated oxidative stress (Pashkow, 2011) fabricate the irreversible changes in chromatin and karyorrhexis which finally leading to necrosis or the apoptosis. Consistent with histological alteration the DAPI staining conforming the ageing induced apoptosis in ventricular myocardium.
Histomorphometrical study revealed that ageing mediated increased size of the ventricular myocytes plays a crucial role in increased ventricular wall thickness. These changes mainly due to increased physical stress in ageing by activation of various endocrine, paracrine, and autocrine factors that activate a variety of receptors (Cooper, 1997; Chien 1999; Chien and Olson, 2002). These receptors trigger multiple cytoplasmic signal transduction cascades that regulate gene expression (Molkentin and Dorn, 2001), resulting in increased cell size due to increased synthesis of sarcomeric and other proteins together with reorganization of myofibrillar structures.
Masson’s trichrome study showing marked increase in the fibrotic tissue in the aged heart than the control. These increased collagen deposition might be due to the ageing induced oxidative stress and the inflammatory reaction (Pashkow, 2011). These altered pathways might activate the fibroblast to synthesis of collagen (Siwik et al., 2001; Cheng et al., 2003) or the replacement (scar formation) mechanism in the apoptotic cardio myocytes (Isoyama and Nitta-Komatsubara, 2002). This increased cardiac fibrosis which disrupts normal cell-to-cell coupling and increases muscle stiffness this is usually reflected by incomplete relaxation during early diastolic filling, and presumably account for the decrease in early left ventricular diastolic compliance. The accumulation of collagen within the myocardium also disturbs the myocardial function which might be the main factor for ageing related ventricular tachycardia and fibrillation (Morita et al., 2009).
From the present study it is hypothesized that the ageing mediated ventricular dysfunction as a result of multi-factorial casus in particular oxidative stress and inflammation induced ventricular cardiac myocyte apoptosis and fibrosis. Hence, it appears plausible that an increased cardiac cell size and increased extracellular collagen might contribute towards impairment of cardiac health and longevity in ageing. The mechanistic pathways for increased age-related collagen accumulation, apoptosis and cell size in the aged hearts will need to be further elucidated.
References
Anversa P, Hiler B, Ricci R, Guideri G, Olivetti G (1986) Myocyte cell loss and myocyte hypertrophy in the aging rat heart. J Am Coll Cardiol, 8:1441-8.
Barouch LA, Cappola TP, Harrison RW, Crone JK, Rodriguez ER, Burnett AL, Hare JM (2003) Combined loss of neuronal and endothelial nitric oxide synthase causes premature mortality and age-related hypertrophic cardiac remodeling in mice. J Mole Cellu Cardiol, 35: 637–644.
Biesiadecki BJ, Tachampa K, Yuan C, Jin JP, de Tombe PP, Solaro RJ (2010) Removal of the cardiac troponin I N-terminal extension improves cardiac function in aged mice. J Biol Chem, 285: 19688–19698.
Calabresi C, Arosio B, Galimberti L, Scanziani E, Bergottini R, Annoni G, Vergani C (2007) Natural aging, expression of fibrosis-related genes and collagen deposition in rat lung. Exp Gerontol, 42:1003-1011.
Cheng TH, Cheng PY, Shih NL, Chen IB, Wang DL, Chen JJ (2003) Involvement of reactive oxygen species in angiotensin II-induced endothelin-1 gene expression in rat cardiac fibroblasts. J Am Coll Cardiol, 42:1845-1854.
Chien KR (1999) Stress pathways and heart failure. Cell, 98: 555-558.
Chien KR, Olson EN (2002) Converging pathways and principles in heart development and disease: CV@CSH. Cell, 26;110:153-162.
Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, Entman ML (2011) Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J Mol Cell Cardiol, 50: 248-256.
Cooper G (1997) Basic determinants of myocardial hypertrophy: a review of molecular mechanisms. Annu Rev Med, 48:13-23.
Folkow B, Svanborg A (1993) Physiology of cardiovascular aging. Physiol Rev, 73:725–764.
Frey N, Olson EN (2003) Cardiac Hypertrophy: The Good, the Bad, and the Ugly. Annu Rev Physiol, 65:45–79.
Gagliano N, Arosio B, Santambrogio D, Balestrieri MR, Padoani G, Tagliabue J, Masson S, Vergani C, Annoni G (2000) Age-dependent expression of fibrosis-related genes and collagen deposition in rat kidney cortex. J Gerontol A Biol Sci Med Sci, 55:B365-372.
Gagliano N, Grizzi F, Annoni G (2007) Mechanisms of aging and liver functions. Dig Dis, 25:118-123.
Glaser J, Stienecker K (2000) Pancreas and aging: a study using ultrasonography. Gerontology, 46:93-96.
Isoyama S, Nitta-Komatsubara Y (2002) Acute and chronic adaptation to hemodynamic overload and ischemia in the aged heart. Heart Fail Rev, 7: 63-69.
Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL (2001). Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol, 87:413-419.
Lakatta EG, Levy D (2003) Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation, 107: 346-354.
Lakatta EG, Sollott SJ (2002) Perspectives on mammalian cardiovascular aging: humans to molecules. Comparative Biochemistry and Physiology – Part A: Molecular & Integrative Physiology, 132: 699–721.
Lakatta EG, Sollott SJ (2002) Perspectives on mammalian cardiovascular aging: humans to molecules. Comparative Biochemistry and Physiology. Part A: Molecular & Integrative Physiology, 132: 699–721.
Ma H, Wang J, Thomas DP, Tong C, Leng L, Wang W, Merk M, Zierow S, Bernhagen J, Ren J, Bucala R, Li J (2010) Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation, 122: 282–292.
Morita N, Sovari AA, Xie Y, Fishbein MC, Mandel WJ, Garfinkel A, Lin SF, Chen PS, Xie LH, Chen F, Qu Z. Weiss JN, and Karagueuzian HS (2009) Increased susceptibility of aged hearts to ventricular fibrillation during oxidative stress. Am J Physiol Heart Circ Physiol, 297: H1594–H1605.
Omoigui S (2007) The Interleukin-6 inflammation pathway from cholesterol to aging – Role of statins, bisphosphonates and plant polyphenols in aging and age-related diseases. Immunity & Ageing, 4:1 doi:10.1186/1742-4933-4-1.
Pashkow FJ (2011) Oxidative Stress and Inflammation in Heart Disease: Do Antioxidants Have a Role in Treatment and/or Prevention? Int J Inflammation, doi:10.4061/2011/514623.
Siwik DA, Pagano PJ, Colucci WS (2001) Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol, 280:C53-60.